Which is the most acidic hydrogen in the following compound?a) ib) iic) iiid) ive) v free expert solution recall that the higher the degree of positive character on hydrogen, the more acidic it is. Acidity, is roughly a measure of the ease with which the compound(s) lose a proton.
Which Is The Most Acidic Hydrogen In The Following Compound. Ethanol (alcohol) is less acidic than ethanoic acid (carboxylic acid) most of the acidic organic. Usually the most acidic hydrogen is around the most electronegative elements like oxygen, fluorine etc. Which will be the most acidic hydrogen in both cases? Which of the following has the most acidic hydrogen?
![Solved:identify The Most Acidic [Ncrerenccs] :Hydrogen In The Following Compounds Using The Table Below: Approximate Pka Values Acidic Hydrogen Pk, Acidic Hydrogen Pk, Sulfonic Acid Alcohol ~15.9 Protonated Alcohol ~2 Acetylene ~25 Solved:identify The Most Acidic [Ncrerenccs] :Hydrogen In The Following Compounds Using The Table Below: Approximate Pka Values Acidic Hydrogen Pk, Acidic Hydrogen Pk, Sulfonic Acid Alcohol ~15.9 Protonated Alcohol ~2 Acetylene ~25](https://cdn.numerade.com/ask_images/9bc11a3c28474937a41a345845eb687d.jpg) Solved:identify The Most Acidic [Ncrerenccs] :Hydrogen In The Following Compounds Using The Table Below: Approximate Pka Values Acidic Hydrogen Pk, Acidic Hydrogen Pk, Sulfonic Acid Alcohol ~15.9 Protonated Alcohol ~2 Acetylene ~25 From numerade.com
Solved:identify The Most Acidic [Ncrerenccs] :Hydrogen In The Following Compounds Using The Table Below: Approximate Pka Values Acidic Hydrogen Pk, Acidic Hydrogen Pk, Sulfonic Acid Alcohol ~15.9 Protonated Alcohol ~2 Acetylene ~25 From numerade.com
Related Post Solved:identify The Most Acidic [Ncrerenccs] :Hydrogen In The Following Compounds Using The Table Below: Approximate Pka Values Acidic Hydrogen Pk, Acidic Hydrogen Pk, Sulfonic Acid Alcohol ~15.9 Protonated Alcohol ~2 Acetylene ~25 :
Amongst the following the compound having the most acidic alpha hydrogen is. According to me, in the first compound 2 should be most acidic as in both 1 and 2, resonance occurs but 2’s carbon is closer to the oxygen, which can stabilize the negative charge on carbon. The acidity of the hydrogen depends on various factors. What makes an alpha hydrogen more acidic?
Acidity, is roughly a measure of the ease with which the compound(s) lose a proton.
The compound with the most acidic hydrogen is. Which is the most acidic hydrogen in the following compound?a) ib) iic) iiid) ive) v free expert solution recall that the higher the degree of positive character on hydrogen, the more acidic it is. Usually the most acidic hydrogen is around the most electronegative elements like oxygen, fluorine etc. As the number of electron withdrawing groups (ewg) increases, the acidic strength also increases as ewg stabilise the carbanion formed after removel of h+ ion. According to me, in the first compound 2 should be most acidic as in both 1 and 2, resonance occurs but 2’s carbon is closer to the oxygen, which can stabilize the negative charge on carbon. Acidity, is roughly a measure of the ease with which the compound(s) lose a proton.
 Source: oneclass.com
Source: oneclass.com
C1−h is most acidic due to the −i effect of c=o. Which will be the most acidic hydrogen in both cases? Ah, hydrogen coming off this carbon hydrogen carbon, all of the hydra jin�s in this compound, our own carbon,.
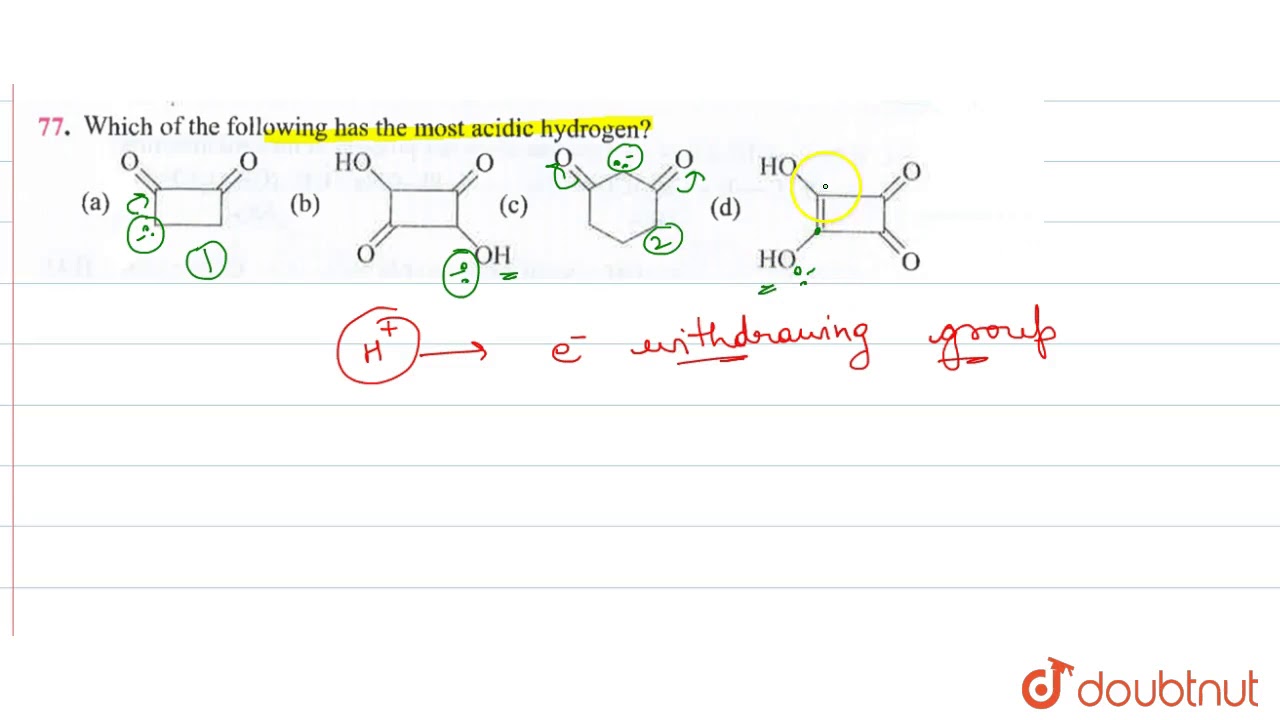 Source: youtube.com
Source: youtube.com
The stability of the structure can be due to conjugation or inductive effect. I ii iv oh o o h h h h h iii v h h h h a) i b) ii c) iii d) iv e) v answer: Which is the most acidic hydrogen in the following compound?

Which of the following, has the most acidic hydrogen? Ethanol (alcohol) is less acidic than ethanoic acid (carboxylic acid) most of the acidic organic. Which one of the following compounds is most acidic?
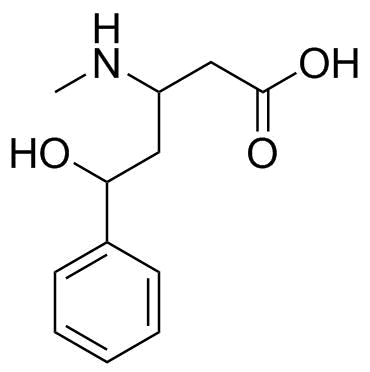 Source: sites.science.oregonstate.edu
Source: sites.science.oregonstate.edu
The compound with the most acidic hydrogen is. Which of the following, has the most acidic hydrogen? Which of the following has the most acidic hydrogen?
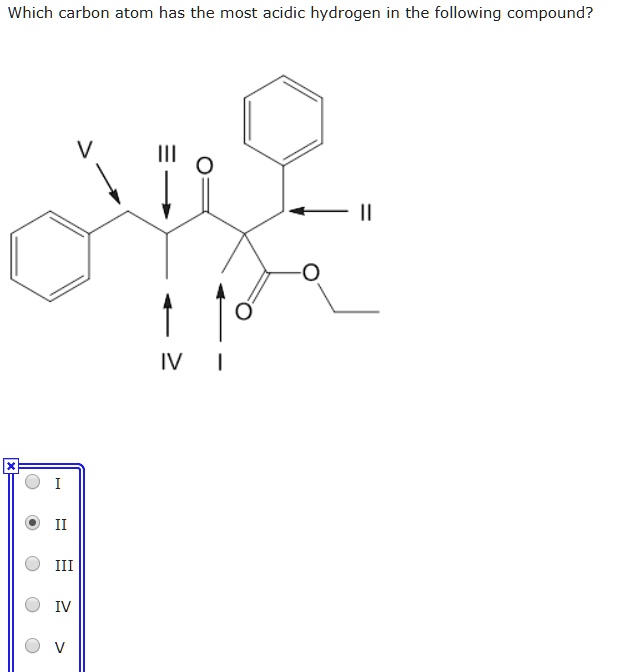 Source: numerade.com
Source: numerade.com
Which is the most acidic hydrogen in the following compound? 0.4 g of polybasic acid hna (all the hydrogens are acidic) requries 0.5 g of naoh for complete neutralisation. What makes an alpha hydrogen more acidic?
![Solved:identify The Most Acidic [Ncrerenccs] :Hydrogen In The Following Compounds Using The Table Below: Approximate Pka Values Acidic Hydrogen Pk, Acidic Hydrogen Pk, Sulfonic Acid Alcohol ~15.9 Protonated Alcohol ~2 Acetylene ~25 Solved:identify The Most Acidic [Ncrerenccs] :Hydrogen In The Following Compounds Using The Table Below: Approximate Pka Values Acidic Hydrogen Pk, Acidic Hydrogen Pk, Sulfonic Acid Alcohol ~15.9 Protonated Alcohol ~2 Acetylene ~25](https://cdn.numerade.com/ask_images/9bc11a3c28474937a41a345845eb687d.jpg) Source: numerade.com
Source: numerade.com
Check the stability of the carbanion formed after removel of most acidic h+ ion. Medium 5 downloaded by liza gonashvili (gonashvili.lia1@gmail.com) 13) which is the most acidic hydrogen in the compound shown? This negative charge can be.
 Source: study.com
Source: study.com
We will consider all the parameters for finding the most acidic hydrogen in the compound. X is an alkyl proton adjacent to a carbonyl. As the number of electron withdrawing groups (ewg) increases, the acidic strength also increases as ewg stabilise the carbanion formed after removel of h+ ion.
 Source: toppr.com
Source: toppr.com
| (4 pts) 3 9 b. Which carbon atom has the most acidic hydrogen in the following compound? Which one of the following compounds is most acidic?

The hydrogen atom which will form a stable resonating structure after its removal will be the most acidic hydrogen atom. Which hydrogen in the following is most acidic? Which of the following has the most acidic hydrogen ?
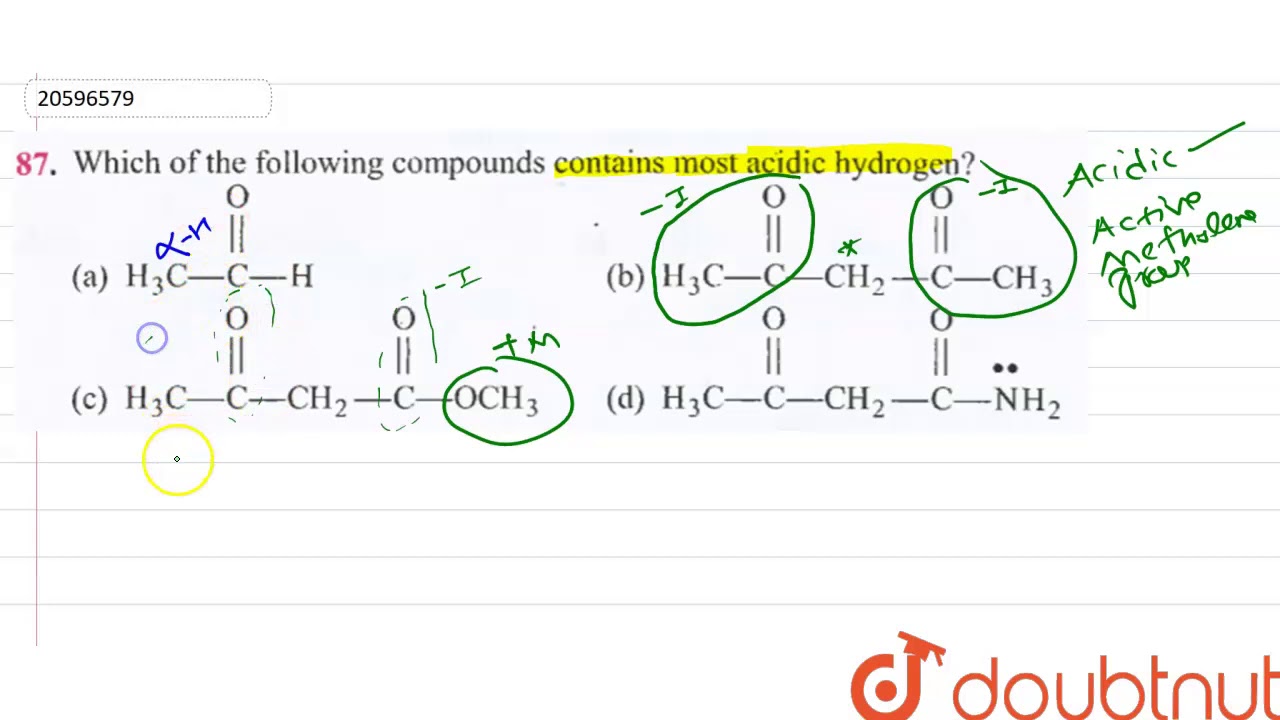 Source: youtube.com
Source: youtube.com
B) heptane (c7h16) sort the alkanes from longest to shortest chain length. Identify the most acidic hydrogen in the following compound. 0=.cn 11 h н iii o iii o iv ov please answer will rate which is the most acidic hydrogen in the following compound?
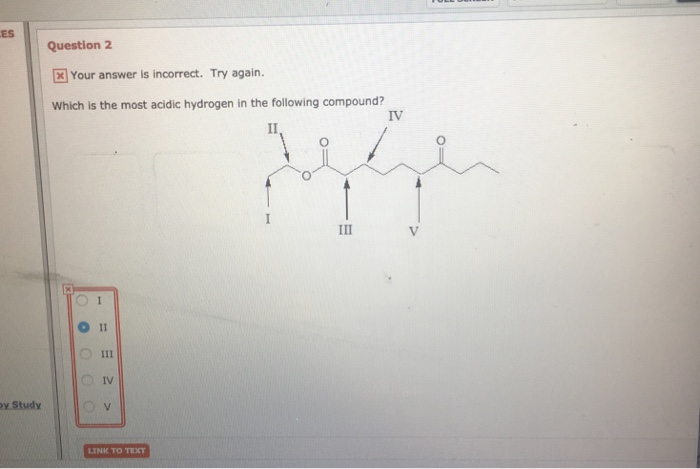
Ah, hydrogen coming off this carbon hydrogen carbon, all of the hydra jin�s in this compound, our own carbon,. Alkyne compounds which have acidic hydrogen atom are more acidic than alkynes which do not have acidic hydrogen atoms. Please answer will rate which is the most acidic hydrogen in the following compound?
 Source: bartleby.com
Source: bartleby.com
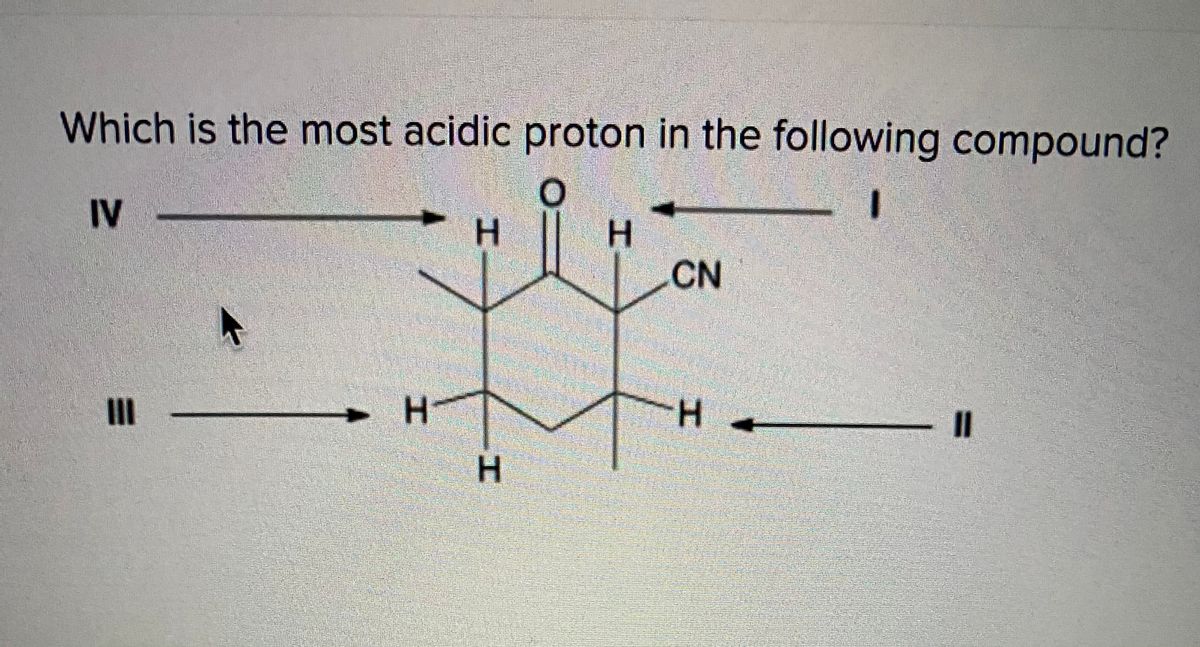
The anion formed after deprotonation is more stable due to the extra resonance structure on the more electronegative oxygen atom. B) heptane (c7h16) sort the alkanes from longest to shortest chain length. X is an alkyl proton adjacent to a carbonyl.
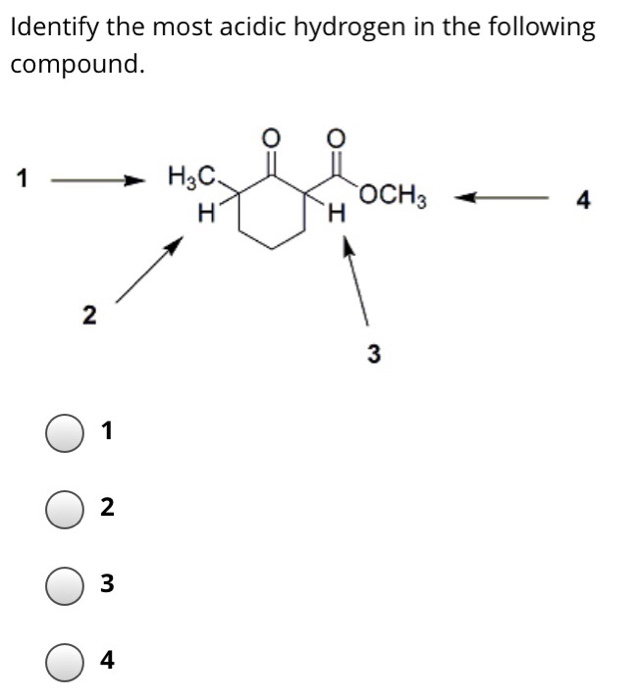 Source: chegg.com
Source: chegg.com
Y is an alkyl proton para to a carbonyl. 0=.cn 11 h н iii o iii o iv ov please answer will rate which is the most acidic hydrogen in the following compound? The hydrogen of phenol and substituted phenols are acidic in general.
 Source: toppr.com
Source: toppr.com
B) heptane (c7h16) sort the alkanes from longest to shortest chain length. | (4 pts) 3 9 b. Please answer will rate which is the most acidic hydrogen in the following compound?
 Source: chemistry.stackexchange.com
Source: chemistry.stackexchange.com
Which of the following has the most acidic hydrogen? Medium 5 downloaded by liza gonashvili (gonashvili.lia1@gmail.com) 13) which is the most acidic hydrogen in the compound shown? Which of the following has the most acidic hydrogen ?
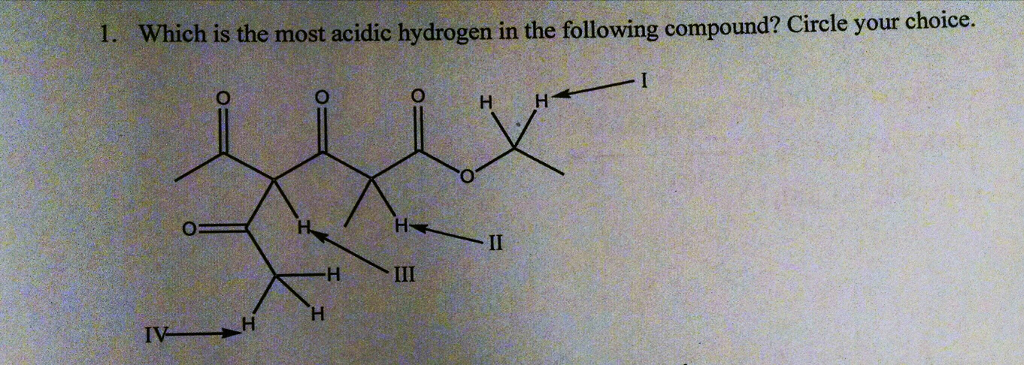 Source: chegg.com
Source: chegg.com
Which of the following has the most acidic hydrogen ? The number of replaceable hydrogen atom asked jun 26, 2019 in chemistry by ranbirpatel ( 67.0k points) Ncert dc pandey sunil batra hc verma pradeep errorless.
 Source: quizlet.com
Source: quizlet.com
Its conjugate base is the weakest base here, and. Aldehydes, ketones and carboxylic acids. C1−h is most acidic due to the −i effect of c=o.
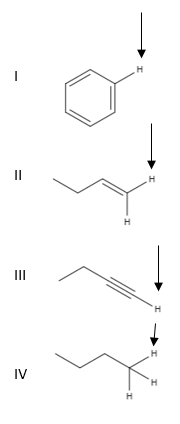 Source: study.com
Source: study.com
Ah, hydrogen coming off this carbon hydrogen carbon, all of the hydra jin�s in this compound, our own carbon,. Carboxylic acid compounds are more acidic than alcohol compounds. The hydrogen of phenol and substituted phenols are acidic in general.
 Source: chegg.com
Source: chegg.com
Few of them are the stability of hydrogen and the electronegativity of the atom it is attached to. Acidity, is roughly a measure of the ease with which the compound(s) lose a proton. Medium 5 downloaded by liza gonashvili (gonashvili.lia1@gmail.com) 13) which is the most acidic hydrogen in the compound shown?
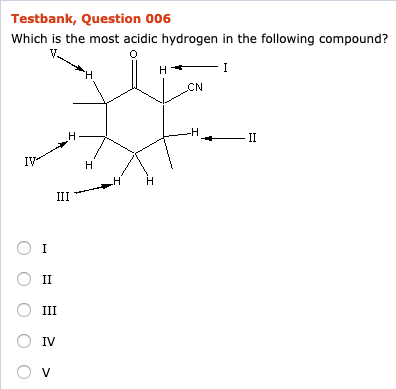 Source: chegg.com
Source: chegg.com
The hydrogen atom which will form a stable resonating structure after its removal will be the most acidic hydrogen atom. Which is the most acidic hydrogen in the following compound? The compound with the most acidic hydrogen is.
Also Read :





