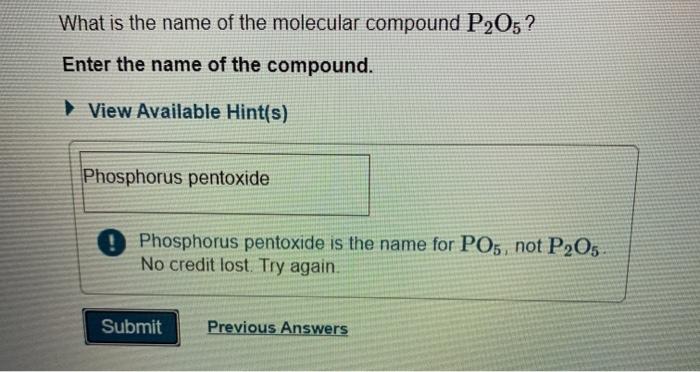
It is a white solid that is used as a desiccant and dehydrating agent. The name for p2o5 is diphosphorus pentoxide.
Which Is The Correct Name For P2o5. What is the correct name for p2o5? Is co no3 3 ionic? Is the correct answer:) hannah is right because the mass of the reactants was different than the mass of the products. It dissolves in strong bases to form the hydroxyplumbate ion, [pb(oh)6]2−:
 The Correct Name For P2O5 Is ____. - Youtube From youtube.com
The Correct Name For P2O5 Is ____. - Youtube From youtube.com
Related Post The Correct Name For P2O5 Is ____. - Youtube :
The correct name for p2o5 is. The numeral “v” signifies the oxidation number of phosphorus , which is +5. Practice until it becomes second nature. What is co no3 2 in chemistry?
Is p2o5 acidic or basic?
Post your question now, it’s free to post, cheap for you, and easy to hire. The correct name for p2o5 is. Phosphorous pentoxide, p 4 o 10 is acidic as it react with water form phosphoric acid. What is the name for p2o5? What is the name of co no3 3? If the garden is 48 feet away, how many days.
 Source: youtube.com
Source: youtube.com
Is a covalent compound because in this compound the sharing of electrons takes place between phosphorous and oxygen. Pbo2 + 2 naoh + 2 h2o → na2[pb(oh)6] what is the ratio of atoms in mgbr2? Post your question now, it’s free to post, cheap for you, and easy to hire.
 Source: youtube.com
Source: youtube.com
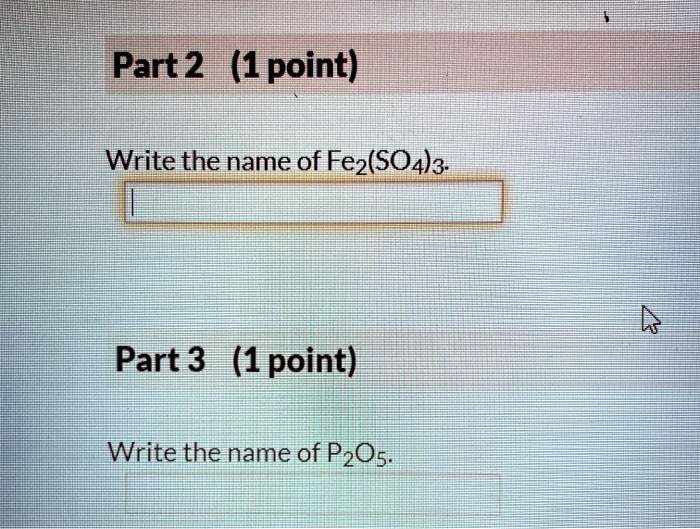
The name of the given formula is diphosphorus pentoxide. The systematic name for p2o5 is phosphorus(v) oxide. The compound’s name however was derived from its empirical formula, not from its molecular formula.
 Source: slideplayer.com
Source: slideplayer.com
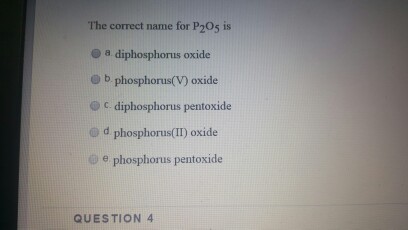
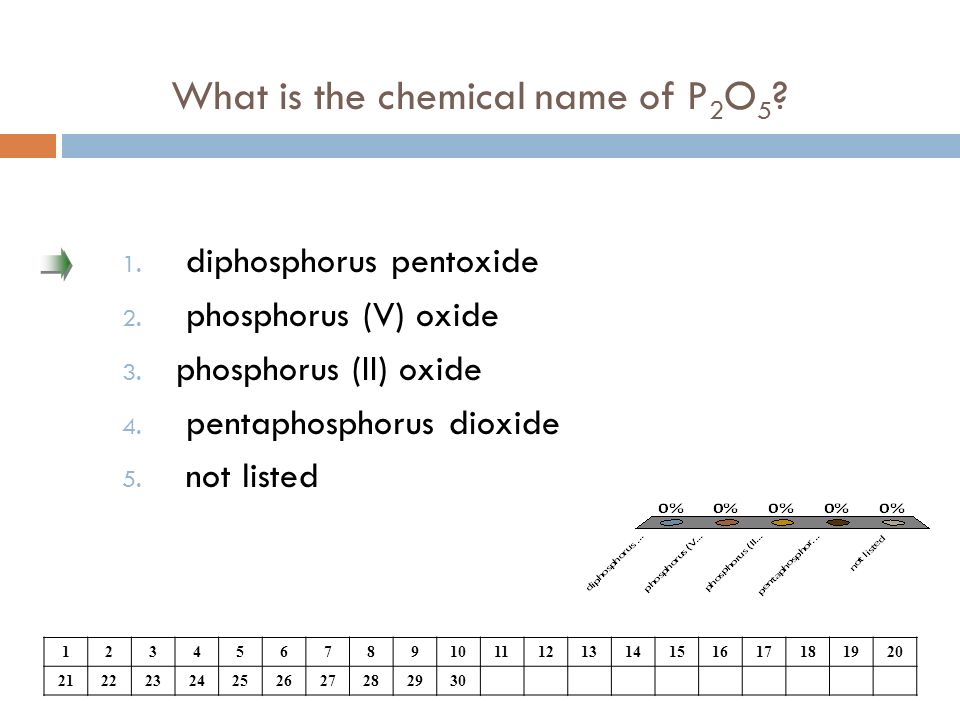
What is the formula for p2o5? What is the correct name for the compound p2o5 brainly? The correct name for p2o5 is group of answer choices diphosphorus oxide phosphorus(v) oxide diphosphorus pentoxide phosphorus pentoxide phosphorus(ii) oxide.
 Source: acronymsandslang.com
Source: acronymsandslang.com
It is a white solid that is used as a desiccant and dehydrating agent. The numeral “v” signifies the oxidation number of phosphorus , which is +5. O phosphorus dioxide o phosphorus pentoxide diphosphorus pentoxidev diphosphorus hexaoxide
 Source: numerade.com
Source: numerade.com
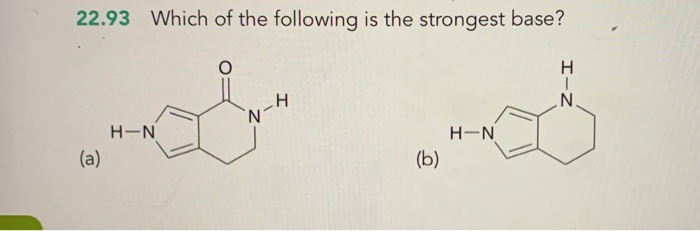
What is the name of co no3 3? Apply the rules for naming that type of compound. What is the name of co no3 3?
 Source: slideplayer.com
Source: slideplayer.com
Which is the correct name for p2o5? Exists in several forms but is often referred to by its empirical formula p2o5. How to write the name for p 2 o 5.
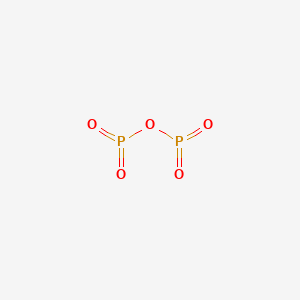
Which is the correct name for p205? Is the correct answer:) hannah is right because the mass of the reactants was different than the mass of the products. Phosphorus pentoxide is a chemical compound with molecular formula p 4 o 10 (with its common name derived from its empirical formula, p 2 o 5).
 Source: slideplayer.com
Source: slideplayer.com
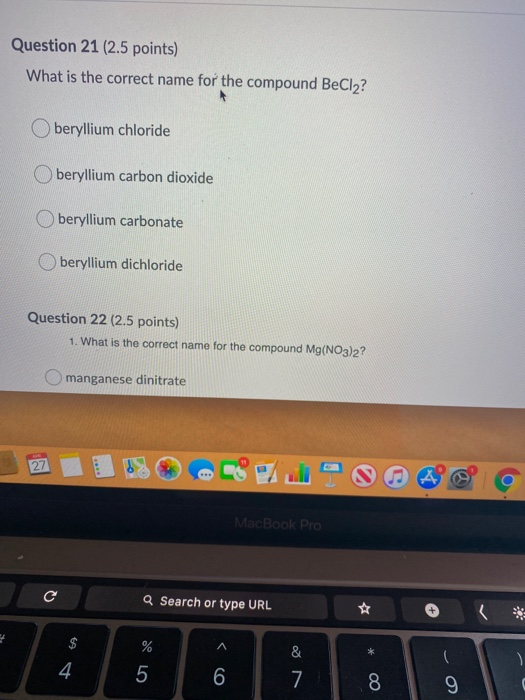
Determine the type of compound you are working with. O phosphorus dioxide o phosphorus pentoxide diphosphorus pentoxidev diphosphorus hexaoxide Which is the correct name for p2o5?
 Source: study.com
Source: study.com
Phosphorous pentoxide, p 4 o 10 is acidic as it react with water form phosphoric acid. Why is p2o5 not diphosphorus? Diphosphorus pentoxide the name of the given formula is diphosphorus pentoxide.

The numeral v signifies the oxidation number of phosphorus, which is +5. Pbo2 + 2 naoh + 2 h2o → na2[pb(oh)6] what is the ratio of atoms in mgbr2? Phosphorus pentoxide is a chemical compound with molecular formula p 4 o 10 (with its common name derived from its empirical formula, p 2 o 5).
 Source: chegg.com
Source: chegg.com
Phosphorus pentoxide is a chemical compound with molecular formula p 4 o 10 (with its common name derived from its empirical formula, p 2 o 5 ). Pbo2 + 2 naoh + 2 h2o → na2[pb(oh)6] what is the ratio of atoms in mgbr2? The standard name for this compound is actually diphosphorus pentoxide.
In the same way p 2o3 is called phosphorus (iii) oxide, as the oxidation number of phosphorus is +3. P 2 o 5 is named diphosphorus pentaoxide. How to write the name for p 2 o 5.
 Source: toppr.com
Source: toppr.com
Which is the correct name for p2o5 quizlet? The systematic name for p 2o5 is phosphorus (v) oxide. The name for p2o5 is diphosphorus pentoxide.
Which is the correct name for p2o5? Correct name for p2o5 correct name polyatomic ions formulas hydrogen phosphate ion Diphosphorus pentoxide the name of the given formula is diphosphorus pentoxide.
 Source: slideplayer.com
Source: slideplayer.com
It dissolves in strong bases to form the hydroxyplumbate ion, [pb(oh)6]2−: Why is p2o5 not diphosphorus? However, the common name for p2o5 or p4h10 is indeed phosphorus pentoxide.
 Source: chegg.com
Source: chegg.com
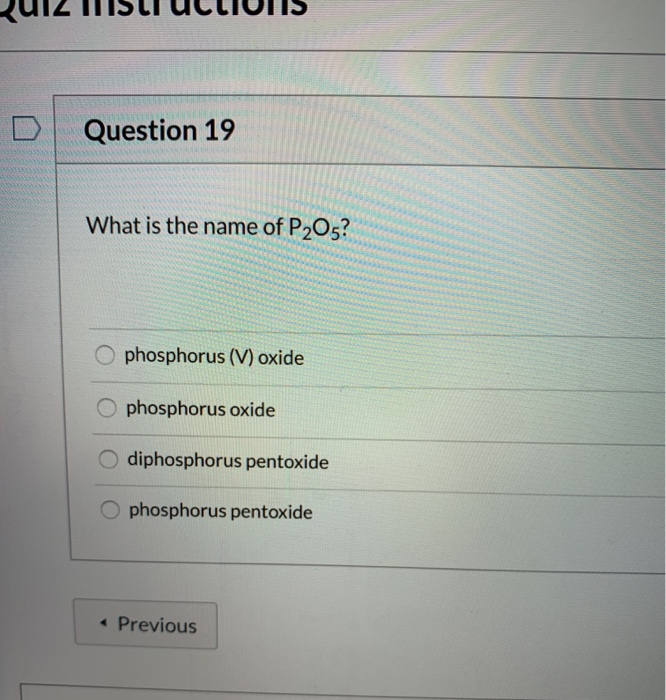
The systematic name for p2o5 is phosphorus(v) oxide. For p 2 o 5 use the hints and resources below to help write the name. The standard name for this compound is actually diphosphorus pentoxide.
 Source: chegg.com
Source: chegg.com
The name for p2o5 is diphosphorus pentoxide. P 2 o 5 is named diphosphorus pentaoxide. What is the compound name for p2o5?
 Source: chegg.com
Source: chegg.com
In the same way p 2o3 is called phosphorus (iii) oxide, as the oxidation number of phosphorus is +3. The systematic name for p 2o5 is phosphorus (v) oxide. In the same way p 2o3 is called phosphorus (iii) oxide, as the oxidation number of phosphorus is +3.
 Source: quorablog.com
Source: quorablog.com
Determine the type of compound you are working with. Is the correct answer:) hannah is right because the mass of the reactants was different than the mass of the products. What is the name for the compound with the formula p2o5?

What happens to ions during bonding to form an ionic compound? Pbo2 + 2 naoh + 2 h2o → na2[pb(oh)6] what is the ratio of atoms in mgbr2? It dissolves in strong bases to form the hydroxyplumbate ion, [pb(oh)6]2−:
Also Read :