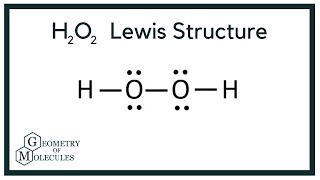
There are a total of 14 valence electrons for h2o2. Valence electrons while each hydrogen will have.
Which Is The Correct Lewis Structure For Hydrogen Peroxide H2o2. We can verify our image is correct because it has. For the h 2 o 2 lewis structure you have 14 valence electrons available. Also, there are two lone pairs on each oxygen atom. Hydrogen peroxide (h2o2) is a very pale blue liquid which appears colorless in a dilute solution, slightly more viscous than water.
 Construct A Lewis Structure For Hydrogen Peroxide, H2O2, In Which Each Atom Achieves An Octet Of - Brainly.com From brainly.com
Construct A Lewis Structure For Hydrogen Peroxide, H2O2, In Which Each Atom Achieves An Octet Of - Brainly.com From brainly.com
Related Post Construct A Lewis Structure For Hydrogen Peroxide, H2O2, In Which Each Atom Achieves An Octet Of - Brainly.com :
Which is the correct lewis structure for hydrogen peroxide, h2o2 3 determine the percentage of hydrogen peroxide in your solution. Draw the lewis structure for hydrogen peroxide, h2o2. To summarize this blog post on h2o2, we can conclude the following: You may also generalize that the oxygens in peroxides have a negative one oxidation state.
The h2o2 lewis structure has a sum of 14 valence electrons.
Which is the correct lewis structure for hydrogen peroxide, h2o2 3 determine the percentage of hydrogen peroxide in your solution. Concept of number of total valence electrons of oxygen and hydrogen atoms are used to draw lewis structure of h 2 o 2. The dihedral angle is affected by hydrogen bonding; Two molecules of hydrogen combine with two molecules of oxygen to form hydrogen peroxide. Hydrogen peroxide is a chemical compound with the formula h2o2. It is important to realize that a carboxylic acid group is not a peroxide.
 Source: techiescientist.com
Source: techiescientist.com
It is a pale blue liquid in its standard state and slowly reacts with. H2o2 or hydrogen peroxide has a quite simple structure, and one can determine its polarity by checking for the net dipole moment in the molecule. We can therefore draw the lewis dot structure as:
 Source: youtube.com
Source: youtube.com
Draw the lewis structure for hydrogen peroxide, h2o2. Hydrogen peroxide is unstable, decomposing readily to oxygen and water with release of heat. Check_circle expert guide draws the structure of lewis peroxide of hydrogen, h2o2.
 Source: clutchprep.com
Source: clutchprep.com
Which is the correct lewis structure for hydrogen peroxide h2o2. Note that the h2o2 lewis structure is frequently used on tests a. It is important to realize that a carboxylic acid group is not a peroxide.
 Source: socratic.org
Source: socratic.org
It is an ionic compound so it would not have a lewis dot structure. Concept of number of total valence electrons of oxygen and hydrogen atoms are used to draw lewis structure of h 2 o 2. 6 + 6 + 1 + 1 = 14 6+6+1+1=14 6 + 6 + 1 + 1 = 14.
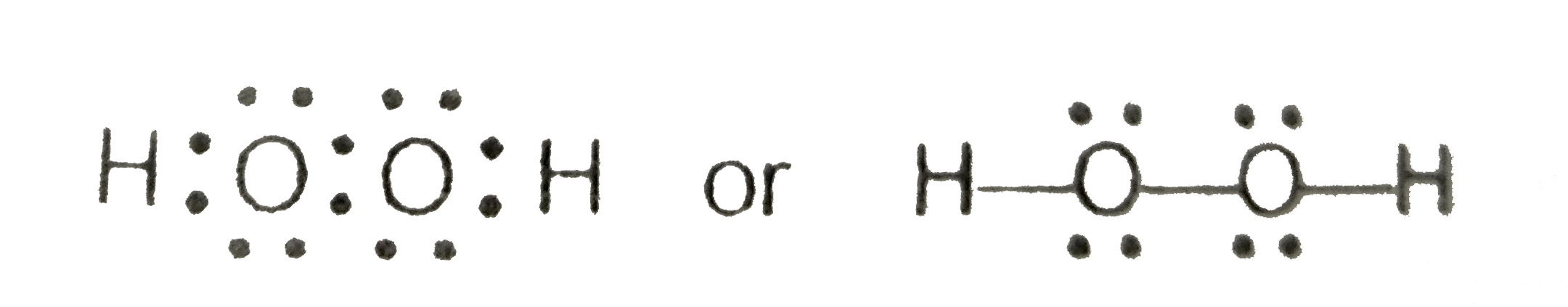 Source: doubtnut.com
Source: doubtnut.com
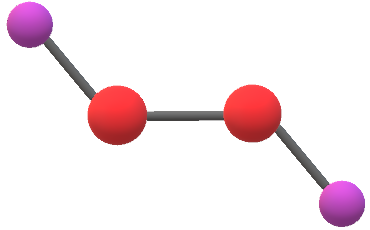
It shows the bonding between the atoms of a molecule and it also shows the unpaired electrons present in the molecule. What molecular shape is h2o2? Calculate the number of moles of o2 produced using the ideal gas law.
 Source: pinterest.com
Source: pinterest.com
6 + 6 + 1 + 1 = 14 6+6+1+1=14 6 + 6 + 1 + 1 = 14. It shows the bonding between the atoms of a molecule and it also shows the unpaired electrons present in the molecule. Here is the correct structure.
 Source: janetcoonce.com
Source: janetcoonce.com
The molecular orbital theory in our syllabus talk only of diatomic molecules so, let me make it clear in that respect. Total number of valence electrons: To summarize this blog post on h2o2, we can conclude the following:
 Source: clutchprep.com
Source: clutchprep.com
The molecular orbital theory in our syllabus talk only of diatomic molecules so, let me make it clear in that respect. Asked by wiki @ 11/06/2021 in chemistry viewed by 61 persons. If yes, then this video can help you find the correct answer here.
 Source: curriculumnacional.cl
Source: curriculumnacional.cl
The h2o2 lewis structure has a sum of 14 valence electrons. E = hc λ, so. The molecular orbital theory in our syllabus talk only of diatomic molecules so, let me make it clear in that respect.
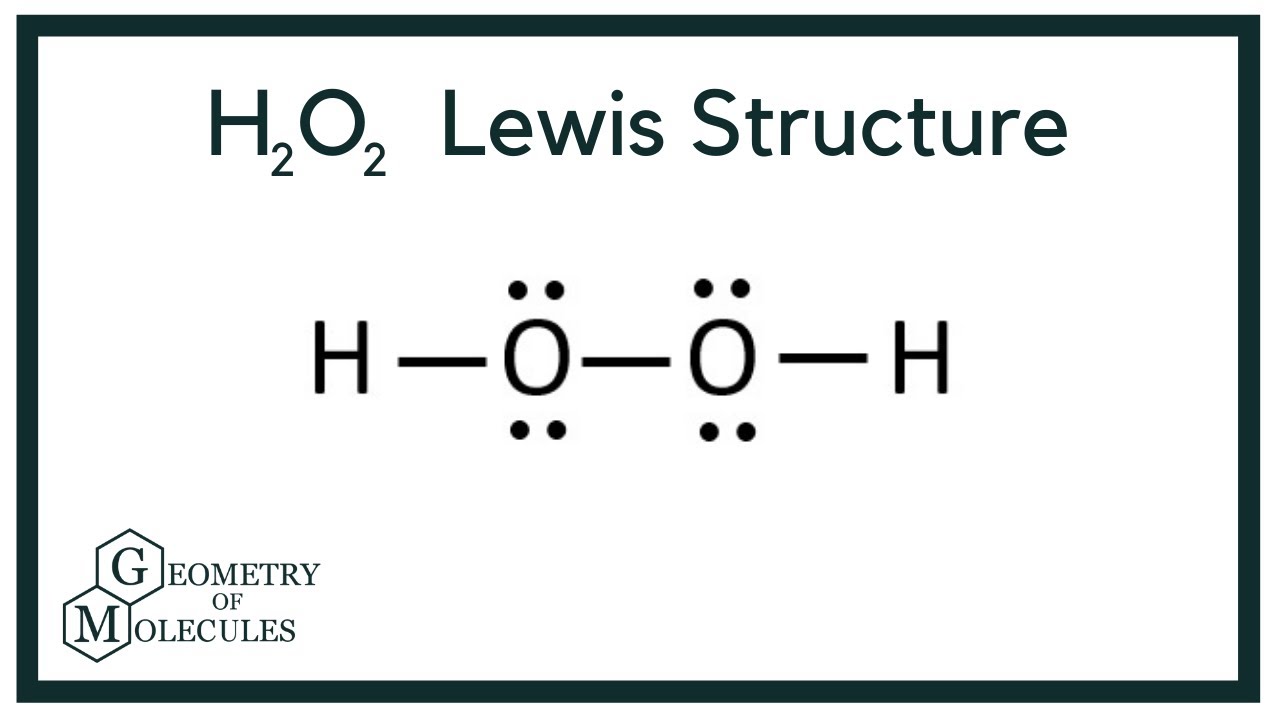
 Source: topblogtenz.com
Source: topblogtenz.com
First determine the total number of valence electrons. It shows the bonding between the atoms of a molecule and it also shows the unpaired electrons present in the molecule. Hydrogen peroxide has 2 types of bonds.
 Source: brainly.com
Source: brainly.com
Never goes in the center and forms only one (1) bond. E = hc λ, so. Note that the h2o2 lewis structure is frequently used on tests a.
 Source: researchgate.net
Source: researchgate.net
Determination of the molecular structure of. (hydrogen peroxide) ever wondered if h2o2 was a polar molecule or a nonpolar one? The valance electrons represented by the �dot�.
 Source: youtube.com
Source: youtube.com
Which is the correct lewis structure for hydrogen peroxide, h2o2 3 determine the percentage of hydrogen peroxide in your solution. H2o2 is a chemical compound with the iupac name hydrogen peroxide. There are a total of 14 valence electrons for h2o2.
 Source: youtube.com
Source: youtube.com
Is h2o2 polar or nonpolar? Hydrogen peroxide has 2 types of bonds. Note that the h2o2 lewis structure is frequently used on tests a.
 Source: brainly.com
Source: brainly.com
The molecular orbital theory in our syllabus talk only of diatomic molecules so, let me make it clear in that respect. Is h2o2 polar or nonpolar? The dihedral angle is 111°.
 Source: study.com
Source: study.com
Hydrogen peroxide is a colorless liquid at room temperature with a bitter taste. In order to continue enjoying our site, we ask you to confirm your identity as a human being. Hydrogen peroxide is unstable, decomposing readily to oxygen and water with release of heat.
 Source: brainly.com
Source: brainly.com
There are a total of 14 valence electrons for h2o2. The molecular orbital theory in our syllabus talk only of diatomic molecules so, let me make it clear in that respect. 6 + 6 + 1 + 1 = 14 6+6+1+1=14 6 + 6 + 1 + 1 = 14.
![Diagram] Lewis Diagrams H2O2 Full Version Hd Quality Diagrams H2O2 - Diagramia.mbreporter.it](https://i.ytimg.com/vi/ca6aKZPBonI/maxresdefault.jpg “Diagram] Lewis Diagrams H2O2 Full Version Hd Quality Diagrams H2O2 - Diagramia.mbreporter.it”) Source: diagramia.mbreporter.it
E = hc λ, so. Hydrogen peroxide has two atoms of hydrogen and two oxygen atoms. As we know that the oxygen has �6� valence electrons and hydrogen has �1� valence electron.
 Source: youtube.com
Source: youtube.com
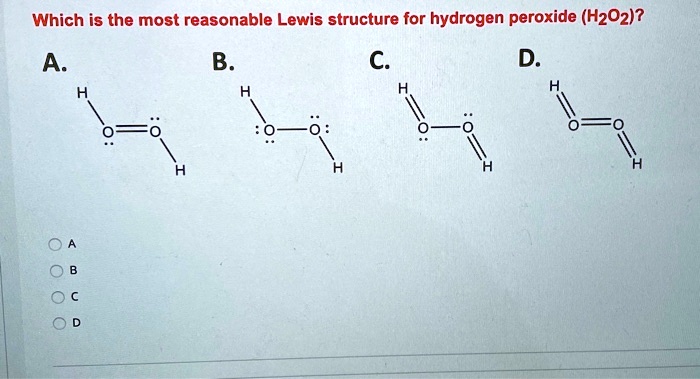
In order to continue enjoying our site, we ask you to confirm your identity as a human being. The dihedral angle is affected by hydrogen bonding; We can verify our image is correct because it has.
 Source: numerade.com
Source: numerade.com
Concept of number of total valence electrons of oxygen and hydrogen atoms are used to draw lewis structure of h 2 o 2. Also, there are two lone pairs on each oxygen atom. Thank you so much for your cooperation.
Also Read :