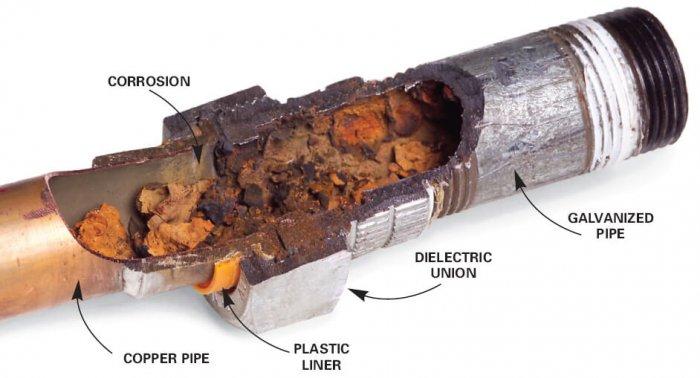
Strong electrostatic force of attraction between the oppositely charged ions, hence, a. Electron pairs are shared among atoms b.
Which Is A Typical Characteristic Of An Ionic Compound. (viii) electrovalent compounds show isomorphism. One atom in the bond has a partial positive charge, while the other atom has a partial negative charge. In the solid state, the positive and negative ions are locked in fixed positions and cannot move freely. They have higher enthalpies of fusion and vaporization than molecular compounds.
 What Is A Typical Characteristic Of An Ionic Compound? | Socratic From socratic.org
What Is A Typical Characteristic Of An Ionic Compound? | Socratic From socratic.org
Related Post What Is A Typical Characteristic Of An Ionic Compound? | Socratic :
Answer choices they form crystals, are very brittle, have high melting points, and conduct electricity when melted Ionic compounds include salts, oxides, hydroxides, sulphides, and the majority of inorganic compounds. (e) poor electrical conductor when molten. High melting and boiling points.
June 15, 2021 by admin.
An ionic bond between oppositely charge ions. The chemical reaction of these compounds are known as ionic reactions, which are fast. Type i and type ii. They conduct electricity when they are dissolved in water. The lithium and chloride ions occupy alternate and opposite positions in the giant lattice structure characteristic of an ionic compound. They have higher enthalpies of fusion and vaporization than molecular compounds.
 Source: brainly.com
Source: brainly.com
The ionic compound has a low solubility in water c. They are hard in nature. Strong electrostatic force of attraction between the oppositely charged ions, hence, a.
 Source: researchgate.net
Source: researchgate.net
The ionic compound has a high melting point which of these elements does not exist as a diatomic molecule? (d) poor electrical conductor when solid. Ionic compounds have high melting and boiling points, again due to strong forces of attraction between the ions.
 Source: slideplayer.com
Source: slideplayer.com
Properties shared by ionic compounds. Calcium chloride (c) state which is not a typical property of an ionic compound. Which is a typical characteristic of an ionic compound?

Which is a typical characteristic of an ionic compound? (a) the chemical formula of ionic compounds has a number subscript which indicates the number of atoms of the ion. In chemistry, an ionic compound is a chemical compound composed of ions held together by electrostatic forces termed ionic bonding.the compound is neutral overall, but consists of positively charged ions called cations and negatively charged ions called anions.these can be simple ions such as the sodium (na +) and chloride (cl −) in sodium chloride, or polyatomic.
 Source: slideplayer.com
Source: slideplayer.com
Calcium chloride (c) state which is not a typical property of an ionic compound. Which is a typical characteristic of an ionic compound? What is a typical characteristic of an ionic compound.
 Source: slideserve.com
Source: slideserve.com
(d) poor electrical conductor when solid. The ionic compound has a low solubility in water c. Strong electrostatic force of attraction between the oppositely charged ions, hence, a.
 Source: socratic.org
Source: socratic.org
The density of the metal is #19.35##g/(cm^3)#. The following properties are all characteristics of ionic compounds except. High melting and boiling points.
 Source: brainly.com
Source: brainly.com
Which is a typical characteristic of an ionic compound? The ionic compound is described as a molecule d. As solids they are almost always electrically insulating, but when melted or dissolved they become highly conductive, because the ions are mobilized.
 Source: slideplayer.com
Source: slideplayer.com
Type i and type ii. What are the four properties of an ionic compound? The lithium and chloride ions occupy alternate and opposite positions in the giant lattice structure characteristic of an ionic compound.
 Source: en.wikipedia.org
Source: en.wikipedia.org
Conduct electricity when dissolved in water. The ionic compound is described as a molecule d. They have high melting points and high boiling points.
 Source: sciencestruck.com
Source: sciencestruck.com
In chemistry, an ionic compound is a chemical compound composed of ions held together by electrostatic forces termed ionic bonding.the compound is neutral overall, but consists of positively charged ions called cations and negatively charged ions called anions.these can be simple ions such as the sodium (na +) and chloride (cl −) in sodium chloride, or polyatomic. What is shown by the structural formula of a molecule? What are the four properties of an ionic compound?
 Source: researchgate.net
Source: researchgate.net
An ionic bond between oppositely charge ions. Conduct electricity when dissolved in water. (b) among the compounds, identify the compound that has all three bonds [ionic, covalent and coordinate bond].
 Source: en.wikipedia.org
Source: en.wikipedia.org
What is a typical characteristic of an ionic compound. The chemical reaction of these compounds are known as ionic reactions, which are fast. Ionic compounds can be categorized into two types.
 Source: slideplayer.com
Source: slideplayer.com
(a) the chemical formula of ionic compounds has a number subscript which indicates the number of atoms of the ion. (b) the formula unit does not give. They are hard in nature.
 Source: slideplayer.com
Source: slideplayer.com
Which is a typical characteristic of an ionic compound? Ionic compounds are hard and rigid due to strong forces of attraction between the oppositely charged ions. The properties of ionic compounds shed some light on the nature of ionic bonds.

They have high melting points and high boiling points. The ionic compound has a low solubility in water c. Ionic compounds can be categorized into two types.

Ionic solids are also poor conductors of electricity for the same reason. Calcium chloride (c) state which is not a typical property of an ionic compound. The ionic compound has a high melting point which of these elements does not exist as a diatomic molecule?
 Source: brainly.com
Source: brainly.com
Ionic compounds dissolve easily in water. In the solid state, the positive and negative ions are locked in fixed positions and cannot move freely. The properties of ionic compounds shed some light on the nature of ionic bonds.
 Source: slideplayer.com
Source: slideplayer.com
(vii) electrovalent compounds furnish ions in solution. Ionic compounds are held by the electrostatic attraction between the atoms. Ionic compounds form when atoms connect to one another by ionic bonds.
 Source: scientific.net
Source: scientific.net
The ionic compound has a high melting point what is shown by the structural formula of a molecule or polyatomic ion? The structure of an ionic compound depends on the relative sizes of the cations and anions. The ionic compound has a high melting point which of these elements does not exist as a diatomic molecule?
Also Read :