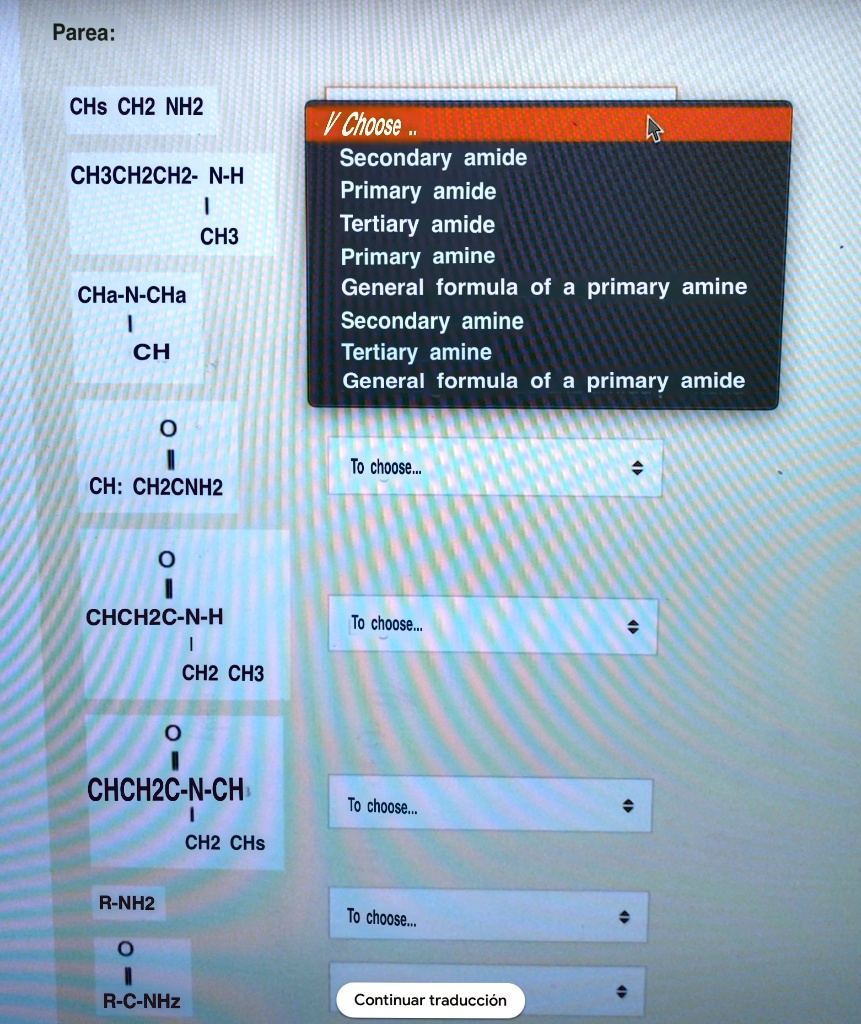
In secondary amine, 2 alkyl groups and one hydrogen atom are directly attached to n atom. Amines have a lone pair so can form a bond with an h⁺ ion when acting as a base.
Which Is A General Representation Of A Secondary Amine. Our collection is the largest in the. (2) amides, like amines, can be classified as primary, secondary, and tertiary. In present case, option c has 2 �h� atoms of nh3 replaced by alkyl group. Enamine offers over 20 000 secondary amines from stock ranging from small decorating building blocks to intermediates and complex scaffolds for library synthesis.
 Which Is A General Representation Of A Secondary Amine?(Pictures From doubtnut.com
Which Is A General Representation Of A Secondary Amine?(Pictures From doubtnut.com
Related Post Which Is A General Representation Of A Secondary Amine?(Pictures :
First, both components are readily deactivated by a transfer of a proton from the acid to the amine and second, the hydroxy unit on the carbonyl of the acid is a relatively poor leaving group. An amine is a derivative of ammonia.it is composed of one or more alkyl groups which replace the hydrogen atoms in ammonia (nh 3) molecule.therefore, the alkyl group is directly bonded to the nitrogen atom. Amines are classified according to the number of carbon atoms bonded directly to the nitrogen atom. Amino acids are organic compounds that contain amino (−nh + 3) and carboxylate −co − 2 functional groups, along with a side chain (r group) specific to each amino acid.
Important representatives include dimethylamine , while an example of an aromatic amine would be diphenylamine.
If two ‘h’ atoms of nh3 are replace by same or different alkyl group, they are referred as secondary amine. In secondary amine, 2 alkyl groups and one hydrogen atom are directly attached to n atom. A primary (1°) amine a compound that has only one alkyl or aryl group on the nitrogen atom. Amino acids are organic compounds that contain amino (−nh + 3) and carboxylate −co − 2 functional groups, along with a side chain (r group) specific to each amino acid. Secondary (2°) amines—secondary amines have two organic substituents (alkyl, aryl or both) bound to the nitrogen together with one hydrogen. Secondary amines still form hydrogen bonds, but having the nitrogen atom in the middle of the chain rather than at the end makes the permanent dipole on the molecule slightly less.
 Source: chegg.com
Source: chegg.com
In addition sulfur (s) is present in the side chains of cysteine and methionine, and selenium (se) in the less. Secondary amines still form hydrogen bonds, but having the nitrogen atom in the middle of the chain rather than at the end makes the permanent dipole on the molecule slightly less. The boiling point of the secondary amine is a little lower than the corresponding primary amine with the same number of carbon atoms.
 Source: quizlet.com
Source: quizlet.com
Has two, and a tertiary (3°) amine a. If one of the �h� atom of nh3 is replace by r = alkyl group, they are referred as primary amine. The boiling point of the secondary amine is a little lower than the corresponding primary amine with the same number of carbon atoms.
 Source: doubtnut.com
Source: doubtnut.com
A primary (1°) amine a compound that has only one alkyl or aryl group on the nitrogen atom. Secondary protein structures are caused by hydrogen bonding between adjacent amine and carboxyl groups. Secondary amines still form hydrogen bonds, but having the nitrogen atom in the middle of the chain rather than at the end makes the permanent dipole on the molecule slightly less.
 Source: sciencedirect.com
Source: sciencedirect.com
If two �h� atoms of nh3 are replace by same or different alkyl group, they are referred as secondary amine. In the usual nomenclature, one adds the term amide to the stem of the parent acid�s name. Secondary (2°) amines—secondary amines have two organic substituents (alkyl, aryl or both) bound to the nitrogen together with one hydrogen.
 Source: doubtnut.com
Source: doubtnut.com
Secondary (2°) amines—secondary amines have two organic substituents (alkyl, aryl or both) bound to the nitrogen together with one hydrogen. In amine amines are classified as primary, secondary, or tertiary depending on whether one, two, or three of the hydrogen atoms of ammonia have been replaced by organic groups. Secondary (2°) amines—secondary amines have two organic substituents (alkyl, aryl or both) bound to the nitrogen together with one hydrogen.
 Source: numerade.com
Source: numerade.com
What structural level is represented by the coiling of the protein chain backbone into an alpha helix? The boiling point of the secondary amine is a little lower than the corresponding primary amine with the same number of carbon atoms. Hence it is secondary amine.
 Source: quizlet.com
Source: quizlet.com
(1) the compound ethylammonium chloride is an amine salt. (1) the compound ethylammonium chloride is an amine salt. Has two, and a tertiary (3°) amine a.
 Source: researchgate.net
Source: researchgate.net
If two ‘h’ atoms of nh3 are replace by same or different alkyl group, they are referred as secondary amine. First, both components are readily deactivated by a transfer of a proton from the acid to the amine and second, the hydroxy unit on the carbonyl of the acid is a relatively poor leaving group. A primary (1°) amine a compound that has only one alkyl or aryl group on the nitrogen atom.
 Source: polymerdatabase.com
Source: polymerdatabase.com
Has two, and a tertiary (3°) amine a. What structural level is represented by the coiling of the protein chain backbone into an alpha helix? (2) amides, like amines, can be classified as primary, secondary, and tertiary.
![⚗️[Please See The Attatched Photo] Which Is A General Representation Of A Secondary Amine? A. An N - Brainly.com ⚗️[Please See The Attatched Photo] Which Is A General Representation Of A Secondary Amine? A. An N - Brainly.com](https://us-static.z-dn.net/files/d46/f64451acdafb46095cb1e9270f0d7886.jpg) Source: brainly.com
Source: brainly.com
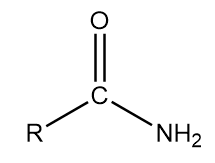
A primary (1°) amine a compound that has only one alkyl or aryl group on the nitrogen atom. Amides are not in general accessible by the direct condensation of amines with carboxylic acids for two reasons: Peptide bonds join the amine group on one amino acid with the r group of another amino acid.
 Source: vedantu.com
Source: vedantu.com
In addition sulfur (s) is present in the side chains of cysteine and methionine, and selenium (se) in the less. Amines are classified according to the number of carbon atoms bonded directly to the nitrogen atom. Has one alkyl (or aryl) group on the nitrogen atom, a secondary (2°) amine a compound that has two alkyl or aryl groups on the nitrogen atom.
 Source: ochempal.org
Source: ochempal.org
Has one alkyl (or aryl) group on the nitrogen atom, a secondary (2°) amine a compound that has two alkyl or aryl groups on the nitrogen atom. (1) the compound ethylammonium chloride is an amine salt. If two ‘h’ atoms of nh3 are replace by same or different alkyl group, they are referred as secondary amine.
 Source: quizlet.com
Source: quizlet.com
If one of the �h� atom of nh3 is replace by r = alkyl group, they are referred as primary amine. Has one alkyl (or aryl) group on the nitrogen atom, a secondary (2°) amine a compound that has two alkyl or aryl groups on the nitrogen atom. Secondary (2°) amines—secondary amines have two organic substituents (alkyl, aryl or both) bound to the nitrogen together with one hydrogen.
 Source: sciencedirect.com
Source: sciencedirect.com
Peptide bonds join the amine group on one amino acid with the r group of another amino acid. In addition sulfur (s) is present in the side chains of cysteine and methionine, and selenium (se) in the less. Amines are classified according to the number of carbon atoms bonded directly to the nitrogen atom.
 Source: commons.wikimedia.org
Source: commons.wikimedia.org
If two ‘h’ atoms of nh3 are replace by same or different alkyl group, they are referred as secondary amine. What structural level is represented by the coiling of the protein chain backbone into an alpha helix? Enamine offers over 20 000 secondary amines from stock ranging from small decorating building blocks to intermediates and complex scaffolds for library synthesis.
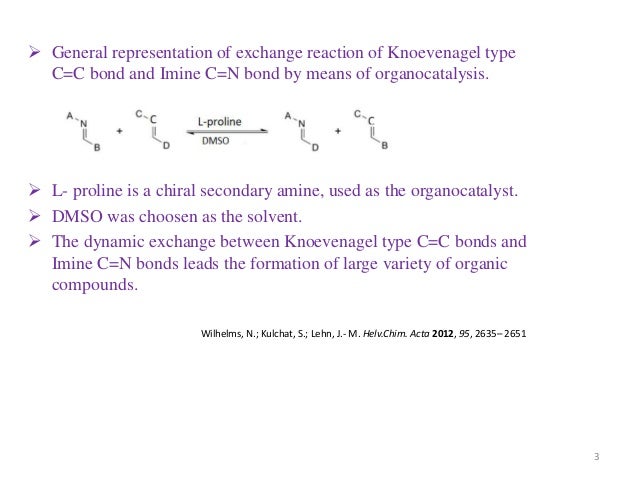 Source: slideshare.net
Source: slideshare.net
It is represented by general formula r 2 n h. In the usual nomenclature, one adds the term amide to the stem of the parent acid�s name. If all the three �h� atom of nh3 are replace by same or different alkyl group, they are referred as tertiary amine.
![Best Answer] Which Is A General Representation Of A Secondary Amine?(Pictures Are In Order) A. - Brainly.com](https://us-static.z-dn.net/files/ddc/dc3de4af6e632cd1a6e39ed2a90cdaf2.jpg “Best Answer] Which Is A General Representation Of A Secondary Amine?(Pictures Are In Order) A. - Brainly.com”) Source: brainly.com
In the usual nomenclature, one adds the term amide to the stem of the parent acid�s name. (2) amides, like amines, can be classified as primary, secondary, and tertiary. Secondary amines still form hydrogen bonds, but having the nitrogen atom in the middle of the chain rather than at the end makes the permanent dipole on the molecule slightly less.
![Best Answer] Which Is A General Representation Of A Secondary Amine?(Pictures Are In Order) A. - Brainly.com](https://us-static.z-dn.net/files/daa/1acabae2c49adac9b129337e3e00eff3.jpg “Best Answer] Which Is A General Representation Of A Secondary Amine?(Pictures Are In Order) A. - Brainly.com”) Source: brainly.com
What is the physical state of most amides. If two ‘h’ atoms of nh3 are replace by same or different alkyl group, they are referred as secondary amine. Rr��n r� 3° amine rhn h 1° amine rhn r� 2° amine hhn h ammonia
 Source: toppr.com
Source: toppr.com
In addition sulfur (s) is present in the side chains of cysteine and methionine, and selenium (se) in the less. Amines are classified according to the number of carbon atoms bonded directly to the nitrogen atom. A primary (1°) amine a compound that has only one alkyl or aryl group on the nitrogen atom.
 Source: ochempal.org
Source: ochempal.org
Amines have a lone pair so can form a bond with an h⁺ ion when acting as a base. Amides are not in general accessible by the direct condensation of amines with carboxylic acids for two reasons: Important representatives include dimethylamine , while an example of an aromatic amine would be diphenylamine.
Also Read :