Addition polymerization one characteristic of the monomers that form condensation polymers that is not common in monomers which form addition polymers is: Which is a characteristic of addition polymerization?
Which Is A Characteristic Of Condensation Polymerization. The polymer contains all the atoms in the monomer units. When two monomers react in a condensation reaction, a small molecule (usually water) is produced as a. Which is a characteristic of addition polymerization? (a) vinyl chloride (b) butadiene (c) styrene (d) all of the above undergoes addition polymerizations.
 10.5: Condensation Polymers - Chemistry Libretexts From chem.libretexts.org
10.5: Condensation Polymers - Chemistry Libretexts From chem.libretexts.org
Related Post 10.5: Condensation Polymers - Chemistry Libretexts :
What is condensation polymerization give an example? Which of these statement are correct for condensation polymerization? Condensation polymerization is a process in which monomers react together to form polymer with the release of small molecules such as water, carbon dioxide etc. Condensation polymers form more slowly than addition polymers, often requiring heat, and they are generally lower in molecular weight.
Condensation polymerization results in the loss of a small molecule, such as water.
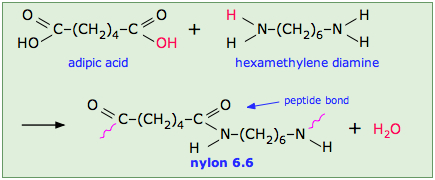
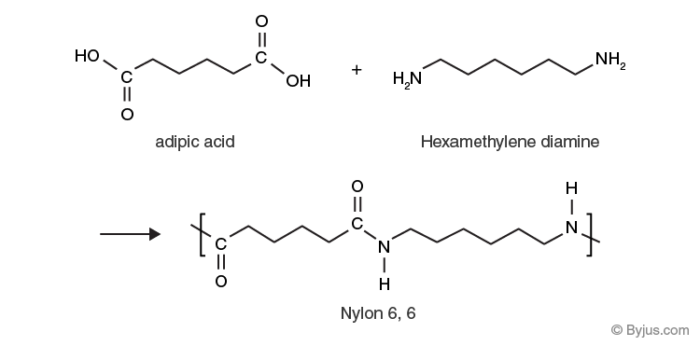
Low thermal expansion is a characteristic or description of most polymeric materials. What is a characteristic of condensation polymerization? Introduction aliphatic polyamides are called nylons. The subunits are joined end to end in long chains. All the atoms in the individual monomers are usually represented. Low thermal expansion is a characteristic or description of most polymeric materials.

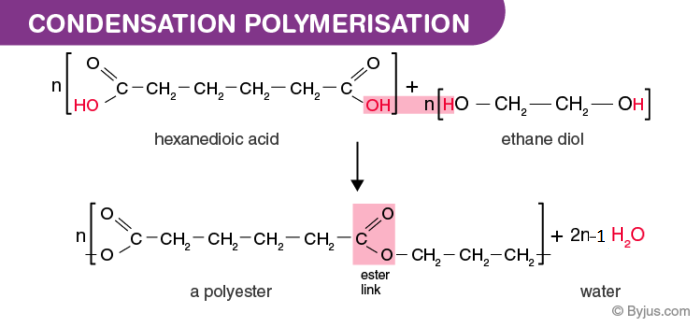
Condensation polymers are any kind of polymers formed through a condensation reaction—where molecules join together—losing small molecules as byproducts such as water or methanol. Condensation polymers are any kind of polymers formed through a condensation reaction—where molecules join together—losing small molecules as byproducts such as water or methanol. Common condensation polymers include polyamides, polyacetals, and proteins.

2 only 3, 1 and 3 only To gain experience in the synthesis of nylon. Condensation polymerization is a process in which monomers react together to form polymer with the release of small molecules such as water, carbon dioxide etc.
 Source: byjus.com
Source: byjus.com
What is condensation polymerization give an example? Polycondensation is a kind of polymerization whose chain growth is based on condensation reaction between two molecules with various degree of polymerization. Condensation polymers are any kind of polymers formed through a condensation reaction—where molecules join together—losing small molecules as byproducts such as water or methanol.
 Source: byjus.com
Source: byjus.com
A typical example of condensation polymers are carbohydrates, nylon 6,6 etc. When two monomers react in a condensation reaction, a small molecule (usually water) is produced as a. (a) vinyl chloride (b) butadiene (c) styrene (d) all of the above undergoes addition polymerizations.
 Source: slideplayer.com
Source: slideplayer.com
The chain can grow from any location where a function group is available. Linear polymers are produced from bifunctional monomers, i.e. Which of the following does not undergo additional polymerization?
 Source: chem.libretexts.org
Source: chem.libretexts.org
To gain experience in the synthesis of nylon. The chain can grow from any location where a function group is available. During addition polymerization, all the atoms in the monomer units are retained in the polymer;

1 and 2 only c. A geneticist has discovered a new compound that is made up of a small number of identical subunits. Polycondensation is a kind of polymerization whose chain growth is based on condensation reaction between two molecules with various degree of polymerization.
 Source:
Source:
A geneticist has discovered a new compound that is made up of a small number of identical subunits. The reaction rarely produces a product. A geneticist has discovered a new compound that is made up of a small number of identical subunits.
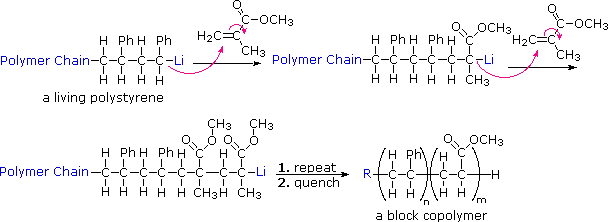 Source: www2.chemistry.msu.edu
Source: www2.chemistry.msu.edu
The chain can grow from any location where a function group is available. Condensation polymers are any kind of polymers formed through a condensation reaction—where molecules join together—losing small molecules as byproducts such as water or methanol. Addition polymerization one characteristic of the monomers that form condensation polymers that is not common in monomers which form addition polymers is:
 Source: sciencedirect.com
Source: sciencedirect.com
Instead of double bonds, these monomers have functional groups (like alcohol, amine, or carboxylic acid groups). To gain experience in the synthesis of nylon. Polycondensation is a kind of polymerization whose chain growth is based on condensation reaction between two molecules with various degree of polymerization.
 Source: collegedunia.com
Source: collegedunia.com
The terminal functional groups on a chain remain active, so that groups of shorter chains combine into longer chains in the late stages of polymerization. The terminal functional groups on a chain remain active, so that groups of shorter chains combine into longer chains in the late stages of polymerization. Addition polymerization one characteristic of the monomers that form condensation polymers that is not common in monomers which form addition polymers is:
 Source: collegedunia.com
Source: collegedunia.com
To understand the characteristic of condensation polymerization. Monomer units are chemically bond together 2. Condensation polymers are any kind of polymers formed through a condensation reaction—where molecules join together—losing small molecules as byproducts such as water or methanol.
 Source: plasticranger.com
Source: plasticranger.com
Linear polymers are produced from bifunctional monomers, i.e. Which is a characteristic of addition polymerization? Condensation polymers form more slowly than addition polymers, often requiring heat, and they are generally lower in molecular weight.
 Source: slideplayer.com
Source: slideplayer.com
Each monomer has more than one functional group. Which of these statement are correct for condensation polymerization? Introduction aliphatic polyamides are called nylons.
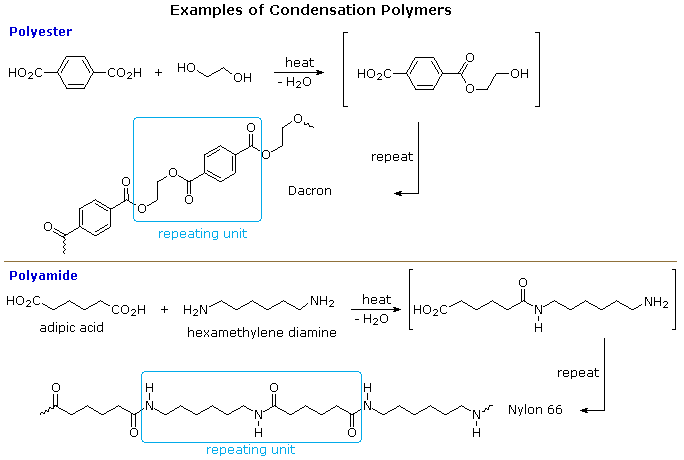 Source: chem.libretexts.org
Source: chem.libretexts.org
The polymer contains all the atoms in the monomer units. The monomers for condensation polymerization have two main characteristics:. The terminal functional groups on a chain remain active, so that groups of shorter chains combine into longer chains in the late stages of polymerization.
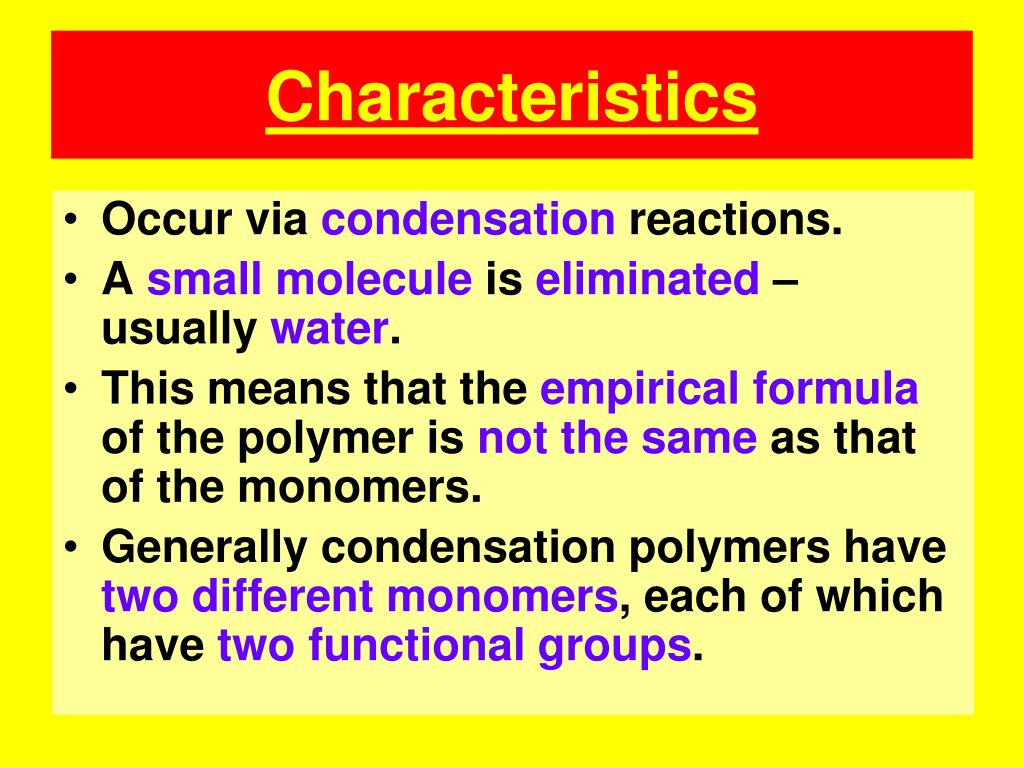 Source: slideserve.com
Source: slideserve.com
The chain can grow from any location where a function group is available. The typical example are polyesters, polyamides and polyethers. 2 only 3, 1 and 3 only
 Source: researchgate.net
Source: researchgate.net
The terminal functional groups on a chain remain active, so that groups of shorter chains combine into longer chains in the late stages of polymerization. Each monomer has more than one functional group. Linear polymers are produced from bifunctional monomers, i.e.
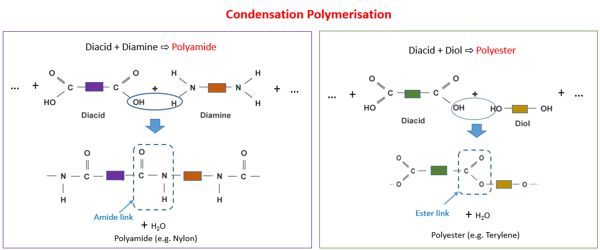 Source: assignmentpoint.com
Source: assignmentpoint.com
Linear polymers are produced from bifunctional monomers, i.e. The polymer contains all the atoms in the monomer units. The polymer contains all the atoms in the monomer units.
 Source: toppr.com
Source: toppr.com
Addition polymerization one characteristic of the monomers that form condensation polymers that is not common in monomers which form addition polymers is: 2 only 3, 1 and 3 only During addition polymerization, all the atoms in the monomer units are retained in the polymer;
 Source: differencebetween.com
Source: differencebetween.com
Linear polymers are produced from bifunctional monomers, i.e. The typical example are polyesters, polyamides and polyethers. It is sometimes confused by condensation previous definition of condensation polymerization.
Also Read :