Because of the ionic link binary ionic compounds have relatively high melting and boiling points. Binary ionic compounds are chemical species that are made of a metal element and a nonmetal element bonding together by an ionic bond.
Which Is A Binary Ionic Compound. Binary ionic compounds are salts which consist of only 2 elements. A binary compound is a substance whose molecules are comprised of atoms of two elements. The key difference between ionic and binary compounds is that ionic compounds contain two charged components whereas binary compounds contain two different chemical elements. Salts. binary ionic compounds are compounds containing only two elements, as demonstrated in the examples below.
 Difference Between Ionic And Binary Compounds | Compare The Difference Between Similar Terms From differencebetween.com
Difference Between Ionic And Binary Compounds | Compare The Difference Between Similar Terms From differencebetween.com
Related Post Difference Between Ionic And Binary Compounds | Compare The Difference Between Similar Terms :
Hydride a(n) _____ is the general name for an ionic compound composed of a cation from a base bonded to an anion produced by an acid. The number of individual atoms in each molecule may vary, but they must belong to only two elements, although isotopes of elements are permitted. There may or may not be more than one of each element. A binary molecular compound made up of hydrogen and a main group element is a(n) _____.
What is the formula of an ionic compound of mg 2+ and i.
Electrolytes are formed when they dissolve in water. Format for naming binary compounds: Binary ionic compounds (type i) the cation (positively charged ion; The key difference between ionic and binary compounds is that ionic compounds contain two charged components whereas binary compounds contain two different chemical elements. When naming these compounds, its composition must be considered. Ionic compounds are binary compounds that come under two different categories.
 Source: youtube.com
Source: youtube.com
An ionic bond is an electrostatic attraction between a. The key difference between ionic and binary compounds is that ionic compounds contain two charged components whereas binary compounds contain two different chemical elements. Hydride a(n) _____ is the general name for an ionic compound composed of a cation from a base bonded to an anion produced by an acid.
 Source: slidetodoc.com
Source: slidetodoc.com
A binary molecular compound made up of hydrogen and a main group element is a(n) _____. A binary compound is a substance whose molecules are comprised of atoms of two elements. What is the formula of an ionic compound of mg 2+ and i.
 Source: slidetodoc.com
Source: slidetodoc.com
Determining binary ionic compound formulas the guiding principle when writing binary ionic compound formulas is that the positive charges on the cation must balance the negative charges on the anion. Salts. binary ionic compounds are compounds containing only two elements, as demonstrated in the examples below. A covalent compound consists of two nonmetal atoms sharing valence electrons.
 Source: youtube.com
Source: youtube.com
A binary compound is one that is made of just two elements. A binary ionic compound is a salt consisting of only two elements in which both elements are ions, a cation and an anion. The key difference between ionic and binary compounds is that ionic compounds contain two charged components whereas binary compounds contain two different chemical elements.
 Source: pinterest.com
Source: pinterest.com
Determining binary ionic compound formulas the guiding principle when writing binary ionic compound formulas is that the positive charges on the cation must balance the negative charges on the anion. A covalent compound consists of two nonmetal atoms sharing valence electrons. In some of these practice questions, you have to figure out the charge of each ion before you can figure out the formula.

Electrostatic attraction holds them together. In a binary ionic compound, the first atom is a metal, while the second atom is a nonmetal. Electrostatic attraction holds them together.
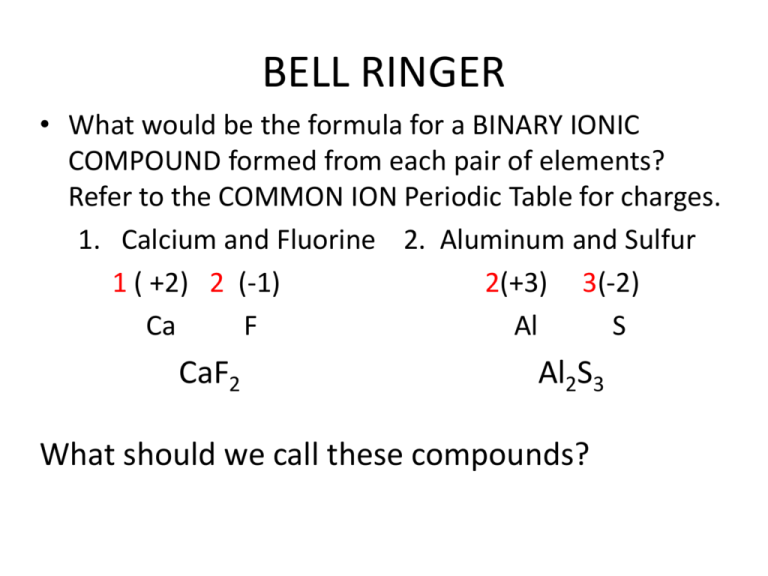 Source: studylib.net
Source: studylib.net
Binary ionic compounds are compounds composed of two ions. The key difference between ionic and binary compounds is that ionic compounds contain two charged components whereas binary compounds contain two different chemical elements. When naming these compounds, its composition must be considered.
 Source: fotokolekcija.blogspot.com
Source: fotokolekcija.blogspot.com
A binary ionic compound is a salt consisting of only two elements in which both elements are ions, a cation and an anion. What is the formula of an ionic compound of mg 2+ and i. An ionic bond is an electrostatic attraction between a.
 Source: slideserve.com
Source: slideserve.com
There may or may not be more than one of each element. In some of these practice questions, you have to figure out the charge of each ion before you can figure out the formula. A covalent compound consists of two nonmetal atoms sharing valence electrons.
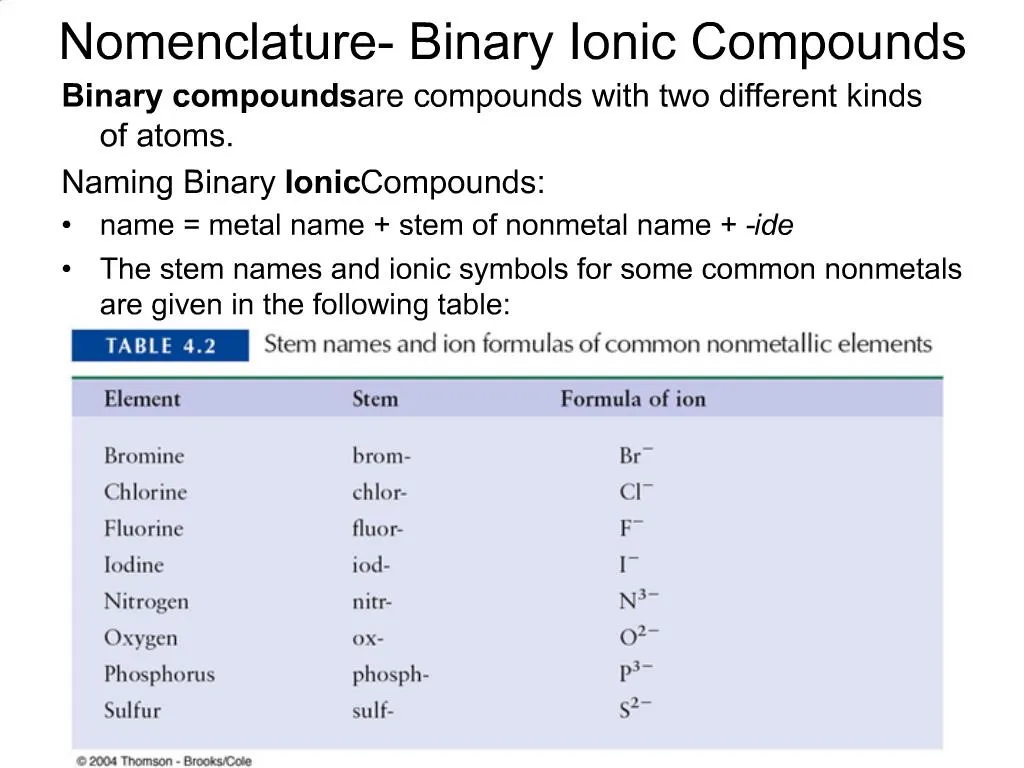 Source: slideserve.com
Source: slideserve.com
Type 1 binary ionic compounds are those in. Contains two elements, both nonmetals. Binary ionic compounds are chemical species that are made of a metal element and a nonmetal element bonding together by an ionic bond.
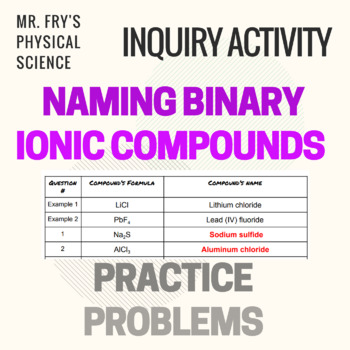 Source: teacherspayteachers.com
Source: teacherspayteachers.com
Therefore, the subscript of the cation is numerically equal to the charge on the anion, vice versa. A binary ionic compound is a salt consisting of only two elements in which both elements are ions, a cation and an anion. Ions are atoms that have a charge when they become part of an ionic compound.

Salts. binary ionic compounds are compounds containing only two elements, as demonstrated in the examples below. A binary ionic compound is a salt consisting of only two elements in which both elements are ions, a cation and an anion. [name of cation (metal)]+ [charge (in parentheses)]+ [base name of anion.
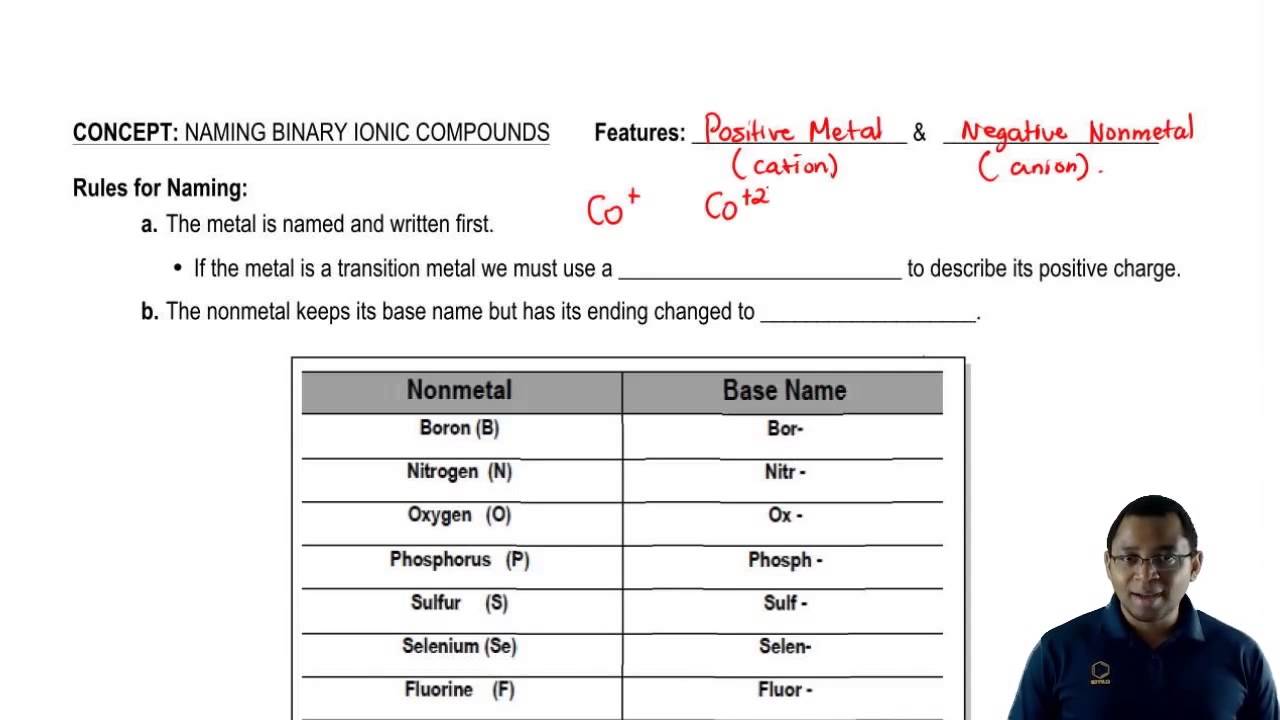 Source: youtube.com
Source: youtube.com
Electrostatic attraction holds them together. An ionic bond is an electrostatic attraction between a. Binary ionic compounds are salts which consist of only 2 elements.
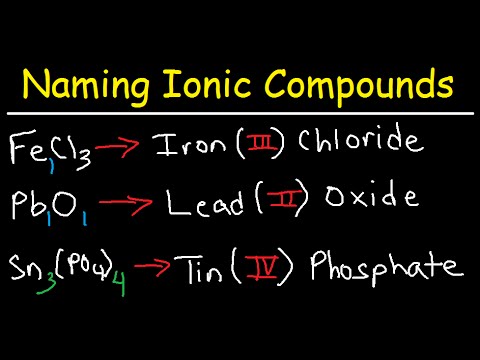 Source: youtube.com
Source: youtube.com
How many atoms do binary ionic compounds have? 246 rows using these games will help you prepare for writing formulas and naming binary. There can be one of each element such as in nacl or kf.
 Source: slideplayer.com
Source: slideplayer.com
A binary ionic compound is a salt consisting of only two elements in which both elements are ions, a cation and an anion. Contains two elements, both nonmetals. When writing formulas for ionic compounds, we use subscripts to indicate how many of each atom is contained in the compound.
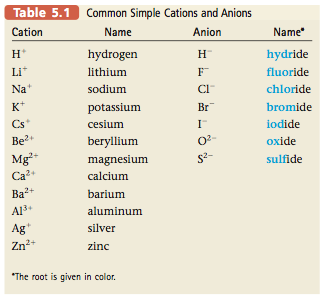 Source: chemistrysaanguyen.weebly.com
Source: chemistrysaanguyen.weebly.com
How many atoms do binary ionic compounds have? Hydride a(n) _____ is the general name for an ionic compound composed of a cation from a base bonded to an anion produced by an acid. Type 1 binary ionic compounds are those in.
 Source: faculty.csupueblo.edu
Source: faculty.csupueblo.edu
Determining binary ionic compound formulas the guiding principle when writing binary ionic compound formulas is that the positive charges on the cation must balance the negative charges on the anion. Cl2 not binary (only one type of atom), but diatomic (two atoms). A covalent compound consists of two nonmetal atoms sharing valence electrons.
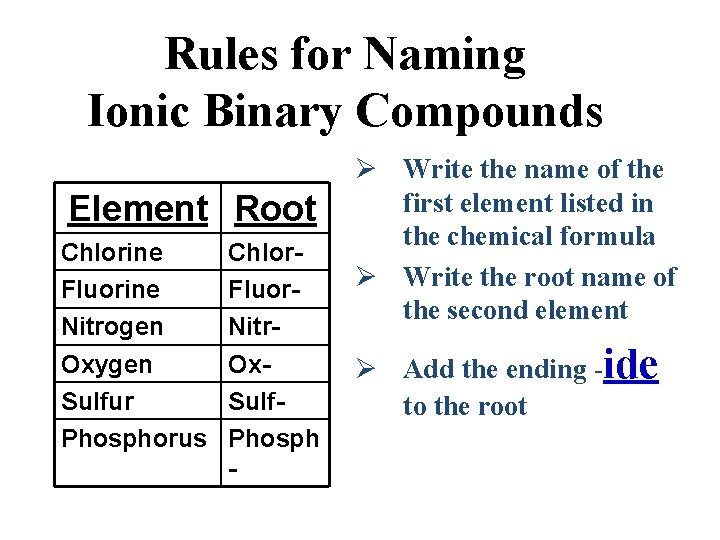 Source: slidetodoc.com
Source: slidetodoc.com
An ionic compound consists of a metal cation bonded to a nonmetal anion. In a binary ionic compound, the first atom is a metal, while the second atom is a nonmetal. The key difference between ionic and binary compounds is that ionic compounds contain two charged components whereas binary compounds contain two different chemical elements.
 Source: sciencenotes.org
Source: sciencenotes.org
Contains two elements, both nonmetals. [name of cation (metal)]+ [charge (in parentheses)]+ [base name of anion. A binary ionic compound is a salt consisting of only two elements in which both elements are ions, a cation and an anion.
 Source: differencebetween.com
Source: differencebetween.com
The binary compound list is mentioned in the table below. Hydride a(n) _____ is the general name for an ionic compound composed of a cation from a base bonded to an anion produced by an acid. An ionic compound consists of a metal cation bonded to a nonmetal anion.
Also Read :





