About 20% of the total carbon buried in marine sediments is organic. In addition to the major constituents, there are numerous minor constituents;.
Which Ions Comprise About 85 Of The Solutes In Seawater. Table 26.1 lists the reference values for blood plasma, cerebrospinal fluid (csf), and urine for the six ions addressed in this section. Animal cells—which lack cell walls—swell and burst if there is a continuous net uptake of water, or shrivel and die if there is a substantial. Wang and chen (1983) found liquid bitterns to be effective and economical coagulants for color removal in pulp and. They come from the atmosphere and dissolves in seawater at the sea surface.
 Pdf) The Major-Ion Composition Of Silurian Seawater From researchgate.net
Pdf) The Major-Ion Composition Of Silurian Seawater From researchgate.net
Related Post Pdf) The Major-Ion Composition Of Silurian Seawater :
Salts derived from seawater evaporation 21 4. Adjustments in respiratory and renal functions allow the body to regulate the levels of these ions in the ecf. Wang and chen (1983) found liquid bitterns to be effective and economical coagulants for color removal in pulp and. Ionic contents resulting from dissolution of modified marine solutes with seawater 58 8.
A solvent is a major component in a solution.
Concept 44.1 osmoregulation balances the uptake and loss of water and solutes. Bicarbonate ions also constitute 48% of river water solutes but only 0.14%. Bicarbonate ions constitute 48% of river water solutes but only 0.14% for seawater. Table 26.1 lists the reference values for blood plasma, cerebrospinal fluid (csf), and urine for the six ions addressed in this section. It consists of water (solvent) and numerous salts (solutes) dissolved in it. Assume that each of the ions in the nacl solution has the same effect on the freezing point of water as a nonelectrolyte molecule, and determine the freezing temperature the solution (which is approximately.
 Source: onlinelibrary.wiley.com
Source: onlinelibrary.wiley.com
Other ions are found in very small concentrations. On the other hand, a solute is a minor component in a solution, for example, sodium salts in seawater. Assume that each of the ions in the nacl solution has the same effect on the freezing point of water as a nonelectrolyte molecule, and determine the freezing temperature the solution (which is approximately.
 Source: slideplayer.com
Source: slideplayer.com
Chemical and physical features of seawater and the world ocean. Animal cells—which lack cell walls—swell and burst if there is a continuous net uptake of water, or shrivel and die if there is a substantial. In most open waters concentrations vary somewhat around.
 Source: researchgate.net
Source: researchgate.net
Bicarbonate ions also constitute 48% of river water solutes but only 0.14%. Ionic contents resulting from dissolution of modified marine solutes with seawater 58 8. Oxygen, carbon dioxide, and nitrogen are typically dissolved in the ocean.
 Source: europepmc.org
Source: europepmc.org
A solvent is a major component in a solution. What are the top 5 elements in seawater? Together, they make up around 85 percent of all dissolved ions in the ocean.
 Source: researchgate.net
Source: researchgate.net
A)ions in solution between the molecules. When protein is found in the urine, that means there is some type of damage to the kidney. Magnesium and sulfate make up another 10 percent of the total.
 Source: rwu.pressbooks.pub
Source: rwu.pressbooks.pub
Concept 44.1 osmoregulation balances the uptake and loss of water and solutes. Bicarbonate ions also constitute 48% of river water solutes but only 0.14%. About 80% of the total particulate carbon flux through the thermocline is in the form of organic matter.
 Source: link.springer.com
Source: link.springer.com
Salts derived from seawater evaporation 21 4. The ions can pass out and the large proteins remain. All animals face the same central problem of osmoregulation.
 Source: researchgate.net
Source: researchgate.net
*although ocean salinities vary from one part of the ocean to another, forchhammer discovered that salt ions are always present in the same proportions. Assume that each of the ions in the nacl solution has the same effect on the freezing point of water as a nonelectrolyte molecule, and determine the freezing temperature the solution (which is approximately. Ionic composition of brines associated with evaporative salt deposition 22 5.
 Source: slideplayer.com
Source: slideplayer.com
Differences like these are due to the varying residence times of seawater solutes; Base your answers to questions 76 through 79 on the information below and on your knowledge of chemistry. Together, they make up around 85 percent of all dissolved ions in the ocean.
 Source: sfamjournals.onlinelibrary.wiley.com
Source: sfamjournals.onlinelibrary.wiley.com
Base your answers to questions 76 through 79 on the information below and on your knowledge of chemistry. Wang and chen (1983) found liquid bitterns to be effective and economical coagulants for color removal in pulp and. Ionic composition of brines associated with evaporative salt deposition 22 5.
 Source: mdpi.com
Source: mdpi.com
Chemical and physical features of seawater and the world ocean. Chemical and physical properties of seawater Seawater contains at least a little of almost everything but the most of the _____ are _____ 85% sodium and chloride account for about ___% of the ions in seawater
 Source: fliphtml5.com
Source: fliphtml5.com
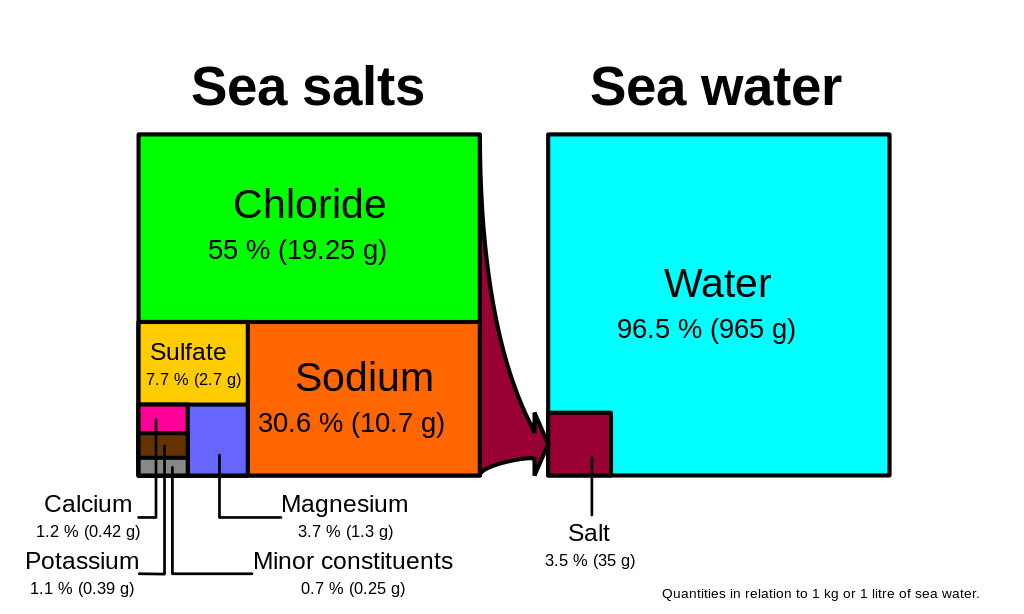
The six most abundant ions of seawater are chloride (cl −), sodium (na +), sulfate (so 2 4 −), magnesium (mg 2+), calcium (ca 2+), and potassium (k +). Chemical and physical properties of seawater Magnesium and sulfate make up another 10 percent of the total.
 Source: slideplayer.com
Source: slideplayer.com
Oxygen, carbon dioxide, and nitrogen are typically dissolved in the ocean. Together, they make up around 85 percent of all dissolved ions in the ocean. Other ions are found in very small concentrations.
 Source: slideshare.net
Source: slideshare.net
In most open waters concentrations vary somewhat around. Base your answers to questions 76 through 79 on the information below and on your knowledge of chemistry. *thus, whether the salinity of a sample of seawater is 32‰ or 37‰, the chloride ion makes up 55.1% of the salt ions in the sample.
 Source: researchgate.net
Source: researchgate.net
About 20% of the total carbon buried in marine sediments is organic. Magnesium and sulfate make up another 10 percent of the total. We study organic matter in seawater because:
 Source:
Source:
Adjustments in respiratory and renal functions allow the body to regulate the levels of these ions in the ecf. In addition to the major constituents, there are numerous minor constituents;. Two of the most prevalent ions in seawater are chloride and sodium.
 Source: researchgate.net
Source: researchgate.net
Hydrogen bonds in water molecules are formed between: Assume that each of the ions in the nacl solution has the same effect on the freezing point of water as a nonelectrolyte molecule, and determine the freezing temperature the solution (which is approximately. Other ions are found in very small concentrations.
 Source: mdpi.com
Source: mdpi.com
Ionic composition of brines associated with evaporative salt deposition 22 5. In most open waters concentrations vary somewhat around. In addition to the major constituents, there are numerous minor constituents;.
 Source: quizlet.com
Source: quizlet.com
The ions can pass out and the large proteins remain. Sodium and chloride comprise over 85% of the seawater solutes explain forchhammer�s principle or the principle of constant proportions the percentage of various salts in seawater in the same in samples from many places, regardless of how salty the water is Other ions are found in very small concentrations.
 Source: sciencedirect.com
Source: sciencedirect.com
Two of the most prevalent ions in seawater are chloride and sodium. Magnesium and sulfate make up another 10 percent of the total. For example, water is the solvent in seawater.
Also Read :





