Ti has atomic number 22 which is not isoelectronic with oxygen anion. Potassium has 19 electrons and 1 is in the outermost shell:
Which Ions Are Isoelectronic With Ar. Which is not isoelectronic with o2 minus? Argon (ar) is an isoelectronic with k+. If playback doesn�t begin shortly, try restarting your device. Ti has atomic number 22 which is not isoelectronic with oxygen anion.
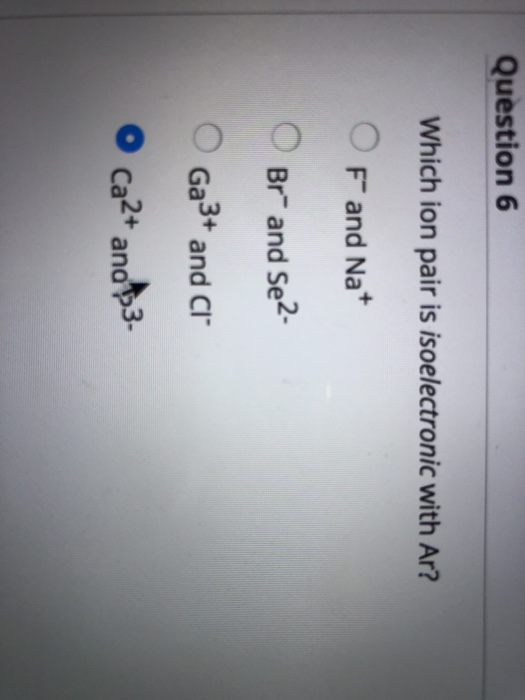 Solved Question 6 Which Ion Pair Is Isoelectronic With Ar? | Chegg.com From chegg.com
Solved Question 6 Which Ion Pair Is Isoelectronic With Ar? | Chegg.com From chegg.com
Related Post Solved Question 6 Which Ion Pair Is Isoelectronic With Ar? | Chegg.com :
A certain elemental ion that is isoelectronic with ar has valence electrons that feel zeff = +6. For example, the k+ ion is isoelectronic with argon. What species are isoelectronic with br? Ti has atomic number 22 which is not isoelectronic with oxygen anion.
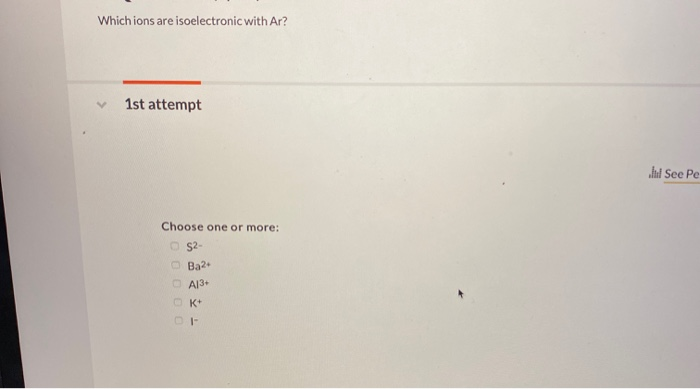
These are such as sulfide ion (s2− ), chloride ion (cl− ) and phosphide ion (p3− ).
Because k+ has the greatest nuclear charge (z = 19), its radius is smallest, and s2− with z = 16 has the largest radius. Asked aug 27, 2019 in chemistry by meisha. Because k+ has the greatest nuclear charge (z = 19), its radius is smallest, and s2− with z = 16 has the largest radius. Isoelectronic means same electronic configuration as ar. For al 3+ would be 10, and for mg 2+ its 10. Argon (ar) is an isoelectronic with k+.
 Source: youtube.com
Source: youtube.com
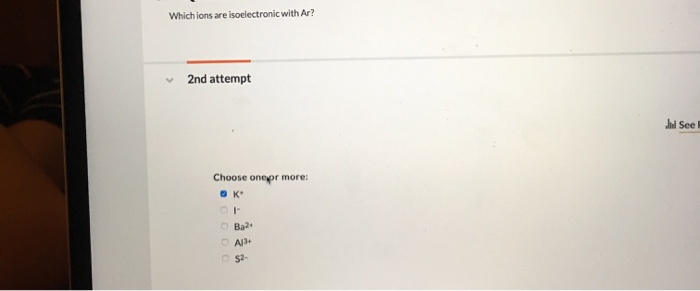
Solved which ions are isoelectronic with ar? Answered aug 27, 2019 by ethann189. As ca +2 contains 18 electrons, all those species containing 18 electrons will be isoelectronic with ca +2.
 Source: clutchprep.com
Source: clutchprep.com
If you are having trouble with chemistry, organic, physics, calculus, or statistics, we got your back! Argon (ar) is an isoelectronic with k+. Which one is not isoelectronic?

Let us help you simplify your studying. For al 3+ would be 10, and for mg 2+ its 10. Potassium has 19 electrons and 1 is in the outermost shell:
 Source: slideplayer.com
Source: slideplayer.com
Fluorine ion is possessing 10 electrons (9 + 1 = 10). Argon (ar) is an isoelectronic with k+. Potassium has 19 electrons and 1 is in the outermost shell:
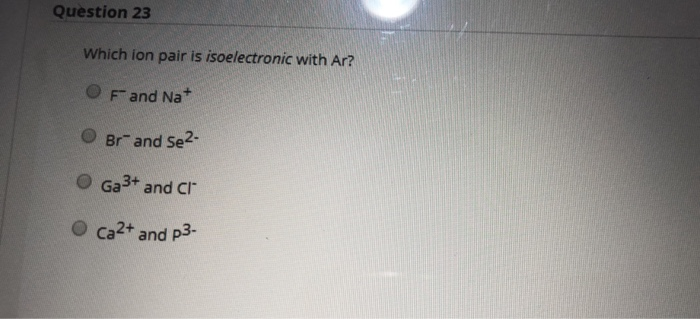 Source: chegg.com
Source: chegg.com
That is, all three ions contain 18 electrons but have different nuclear charges. Our videos prepare you to succeed in your college classes. (ii) ar has 18 electrons.
 Source: sliderbase.com
Source: sliderbase.com
The potassium ion consists of 18 electrons. For example, the k+ ion is isoelectronic with argon. Answered aug 27, 2019 by ethann189.
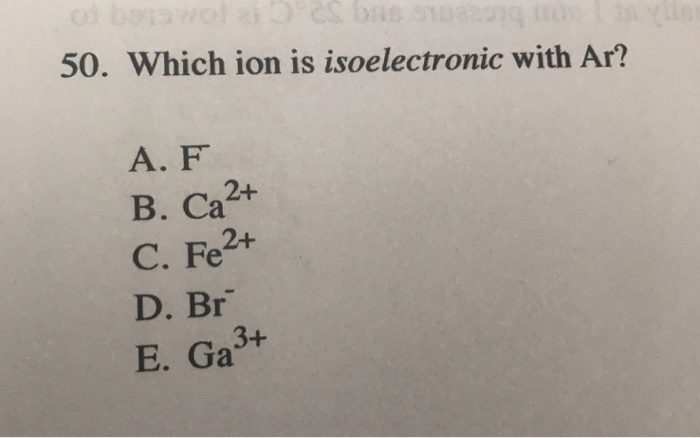 Source: minimalis.co.id
Source: minimalis.co.id
Na+ ca2 + | chegg.com. If you are having trouble with chemistry, organic, physics, calculus, or statistics, we got your back! That is, all three ions contain 18 electrons but have different nuclear charges.
 Source: youtube.com
Source: youtube.com
Bromine with an atomic number of 35 acquires one electron to attain 36 electrons. As ca +2 contains 18 electrons, all those species containing 18 electrons will be isoelectronic with ca +2. What is the ion charge on this ion?
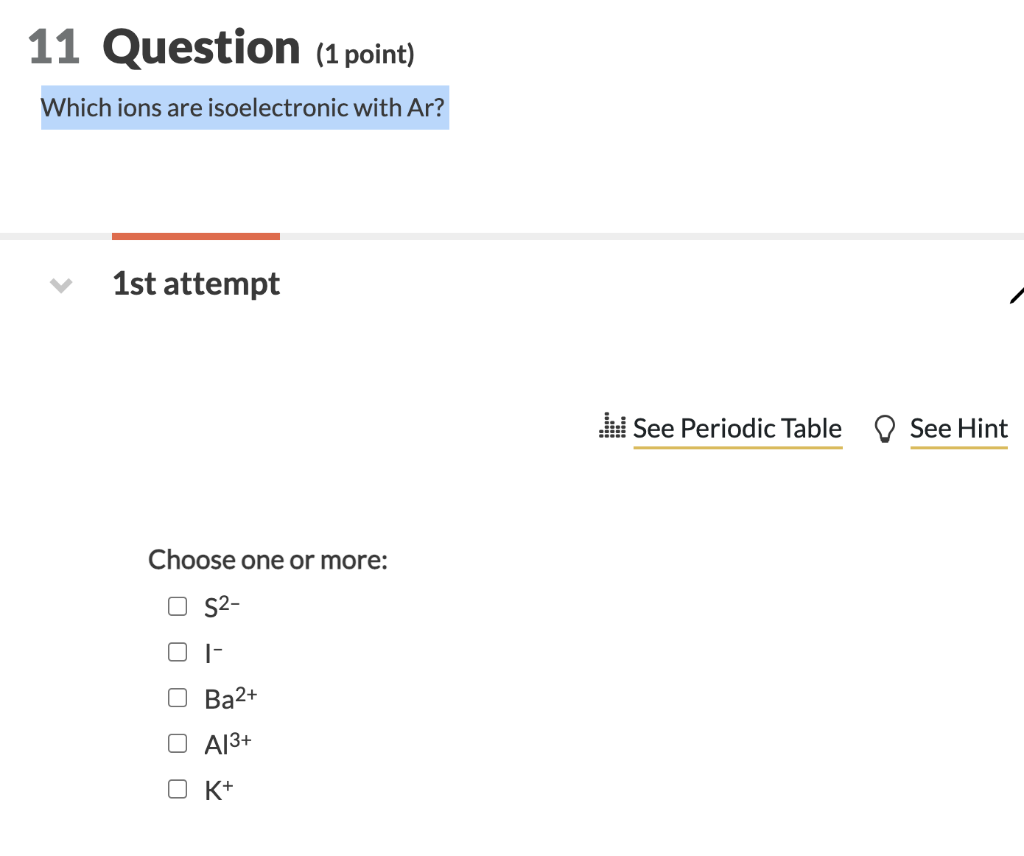
Argon (ar) is an isoelectronic with k+. More than one of the above. Our videos prepare you to succeed in your college classes.
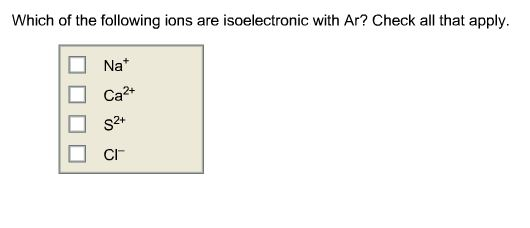 Source: chegg.com
Source: chegg.com
They have the same electron configuration as argon, which means they are isoelectronic. Argon (ar) is an isoelectronic with k+. An isoelectronic series is a group of atoms/ions that have the same number of electrons.
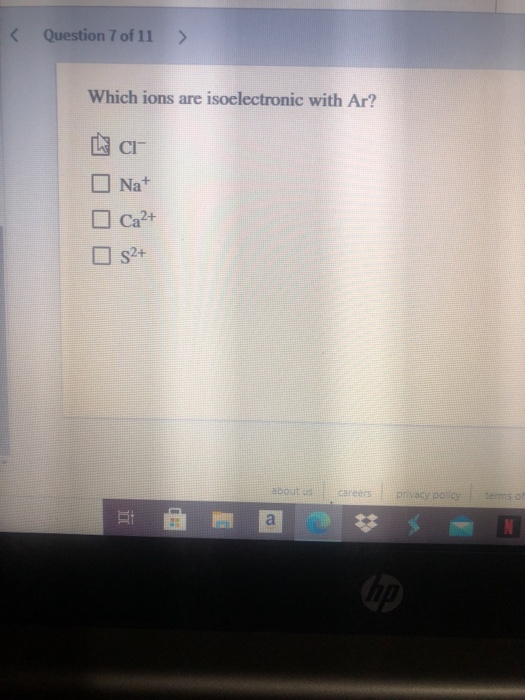 Source: chegg.com
Source: chegg.com
Since, where possible, elements form ions with the same number of electrons as a noble gas, typically these are the numbers of electrons in isoelectronic ions. For example, the k+ ion is isoelectronic with argon. This makes it isoelectronic with krypton.
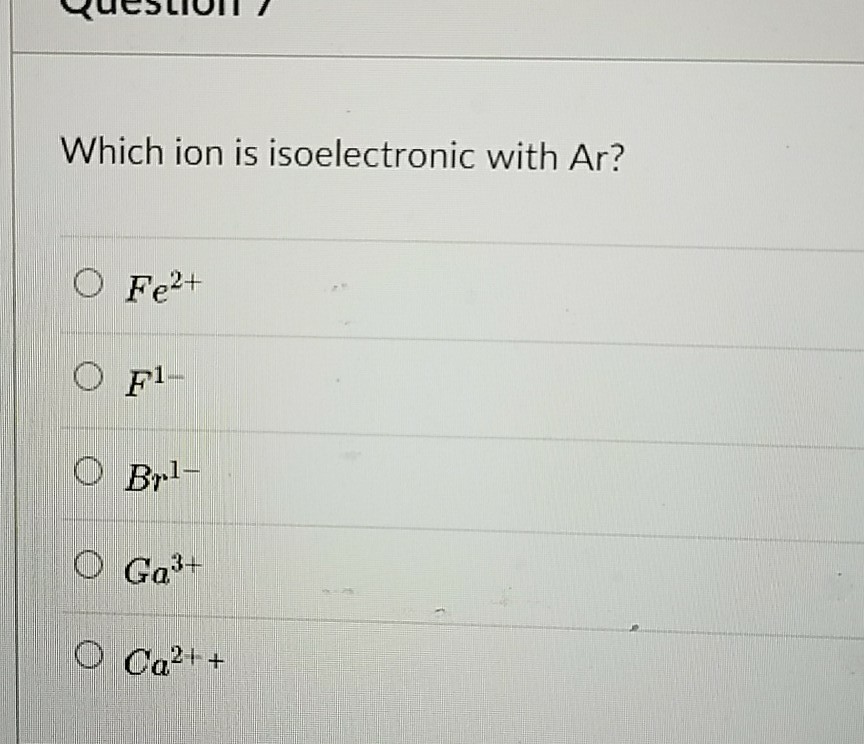
Which ion is isoelectronic with ar? The potassium ion consists of 18 electrons. Answered aug 27, 2019 by ethann189.
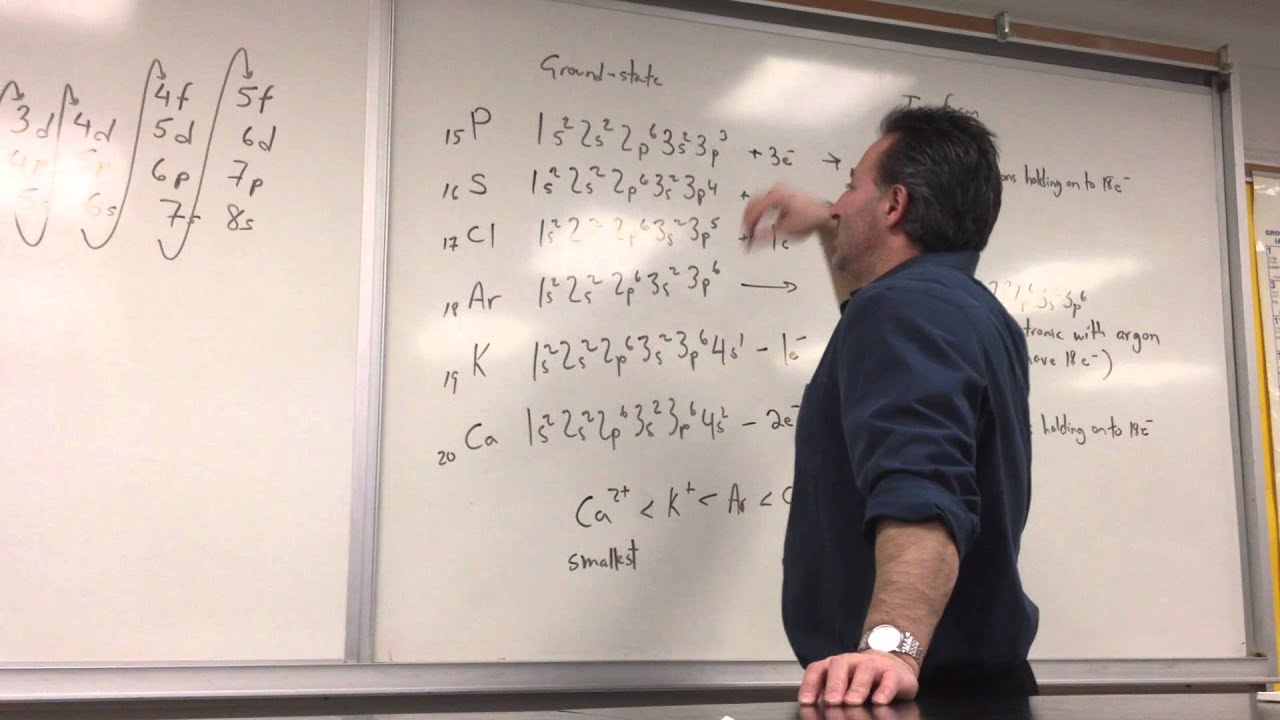 Source: youtube.com
Source: youtube.com
Which ion is isoelectronic with ar? An isoelectronic series is a group of atoms/ions that have the same number of electrons. Which of these species makes an isoelectronic pair:
 Source: chegg.com
Source: chegg.com
Thus, the species isoelectronic with it will also have 18 electrons. Fluorine ion is possessing 10 electrons (9 + 1 = 10). A typical question about isoelectronic series usually involve size comparisons.
 Source: kentchemistry.com
Source: kentchemistry.com
Na +, mg 2+ 1s2 2s2 2p6: These are such as sulfide ion (s2− ), chloride ion (cl− ) and phosphide ion (p3− ). What species are isoelectronic with br?
 Source: chegg.com
Source: chegg.com
Argon is a noble gas and has 18 electrons. Hence o2 is not isoelectronic with other three. Our videos prepare you to succeed in your college classes.

The potassium ion consists of 18 electrons. That is, all three ions contain 18 electrons but have different nuclear charges. Because k+ has the greatest nuclear charge (z = 19), its radius is smallest, and s2− with z = 16 has the largest radius.
 Source: quora.com
Source: quora.com
Na+ ca2 + | chegg.com. (iii) as mg 2+ has 10 electrons, therefore n 3+ f , ne, na + , al 3+ are isoelectric with it. This makes it isoelectronic with krypton.
 Source: chegg.com
Source: chegg.com
Because k+ has the greatest nuclear charge (z = 19), its radius is smallest, and s2− with z = 16 has the largest radius. This ion could be formed from which atom? A certain elemental ion that is isoelectronic with ar has valence electrons that feel zeff = +6.
 Source: youtube.com
Source: youtube.com
Which ion is isoelectronic with ar? Argon (ar) is an isoelectronic with k+. A typical question about isoelectronic series usually involve size comparisons.
Also Read :