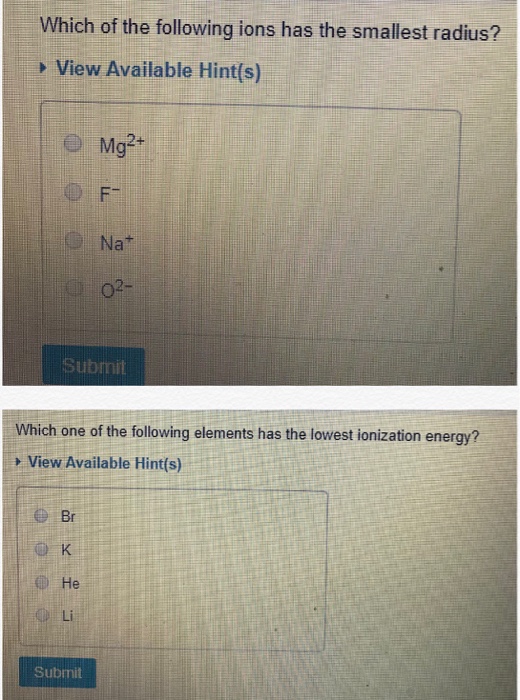
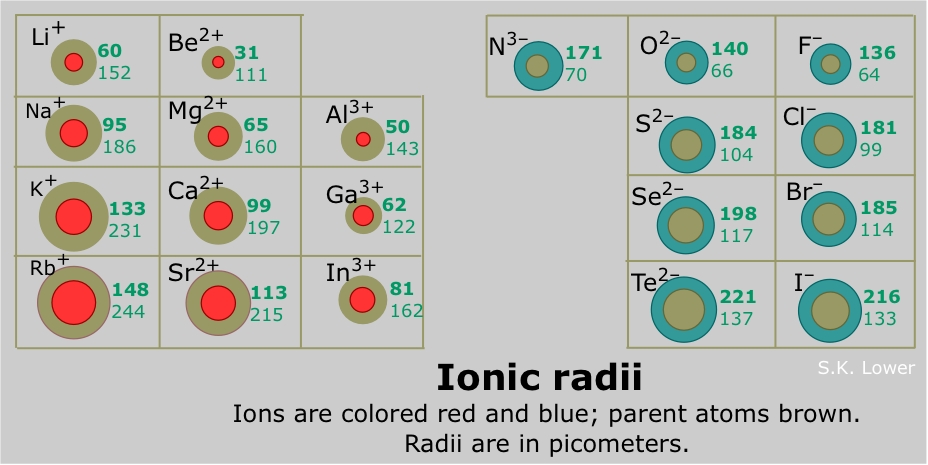
Hence, the ionic radius of mg2+ is smallest. In addition chemistry and technical terms are linked to their definitions in the site's chemistry and.
Which Ion Has The Smallest Ionic Radius. Helium has the smallest atomic radius. Size of mg is less than that of li because of the zeff of mg. Sodium is a group 1 element, so its only ionic state is na+. Cations of a given element have a smaller radius than the neutral atom so that na+ will decrease in size compared with the na atom.
Related Post Solved Which Of The Following Ions Has The Smallest Radius? | Chegg.com :
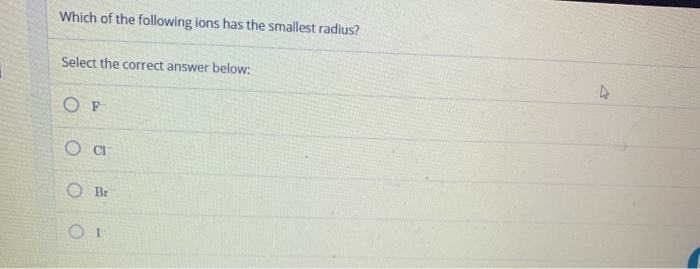
Which element has a smaller ionic radius than magnesium mg brainly? Cations of a given element have a smaller radius than the neutral atom so that na+ will decrease in size compared with the na atom. Which one of the following ion has smallest radius? It may be defined as the effective distance from the nucleus of the ion to the point to which it has an influence in the ionic bond.
Hence, the ionic radius of mg2+ is smallest.
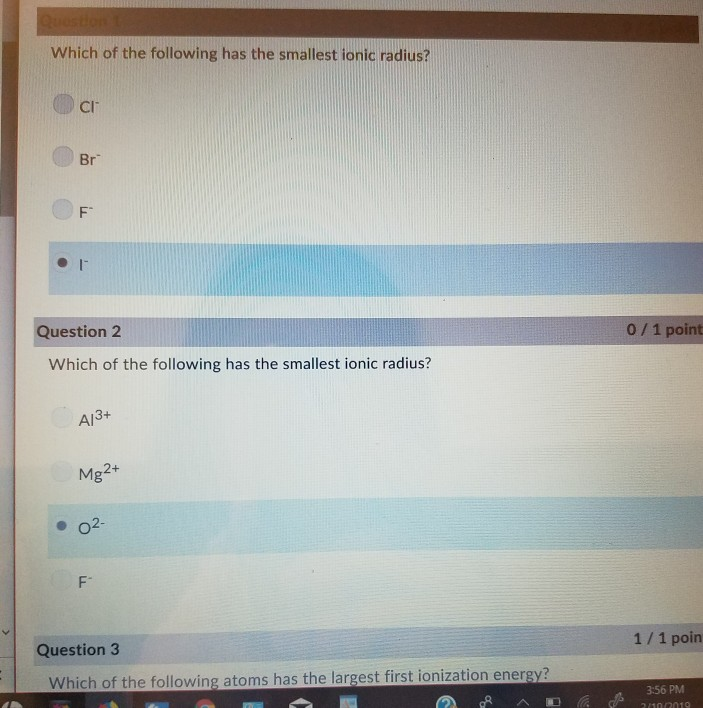
Which of the following has the smallest ionic radii? The cation , which is an ion with a positive charge, by. Which ion has the smallest radius?a. Correct option is c) l i +, b e 2 + a n d b 3 + are isoelectronic species. B e 2 + c. Which ions have the smallest radius?
 Source: chegg.com
Source: chegg.com
Consequently, the ion with the greatest nuclear charge (al3+) is the smallest, and the ion with the smallest nuclear charge (n3−) is the largest….ionic radii and isoelectronic series. Thus, helium is the smallest element, and francium is the largest. Which is bigger li or mg?
Which of the following has the smallest ionic radii? So, in the end, we can conclude that nickel ions have a minimum radius. Which element’s ionic radius is smaller?
 Source: socratic.org
Source: socratic.org
Sodium atoms and sodium ions have the same number of protons. As the protons are added, electrons are attracted with a greater strength towards the core, making the radius of mg smaller. Cations of a given element have a smaller radius than the neutral atom so that na+ will decrease in size compared with the na atom.
 Source: chegg.com
Source: chegg.com
It may be defined as the effective distance from the nucleus of the ion to the point to which it has an influence in the ionic bond. The smallest radius of b 3 + is due to the fact that the high nuclear charge of +3 would be pulling the electron density towards itself more strongly than a +2 or +1. So therefore, elements with a positive charge, will have a smaller ionic radius.
 Source: clutchprep.com
Source: clutchprep.com
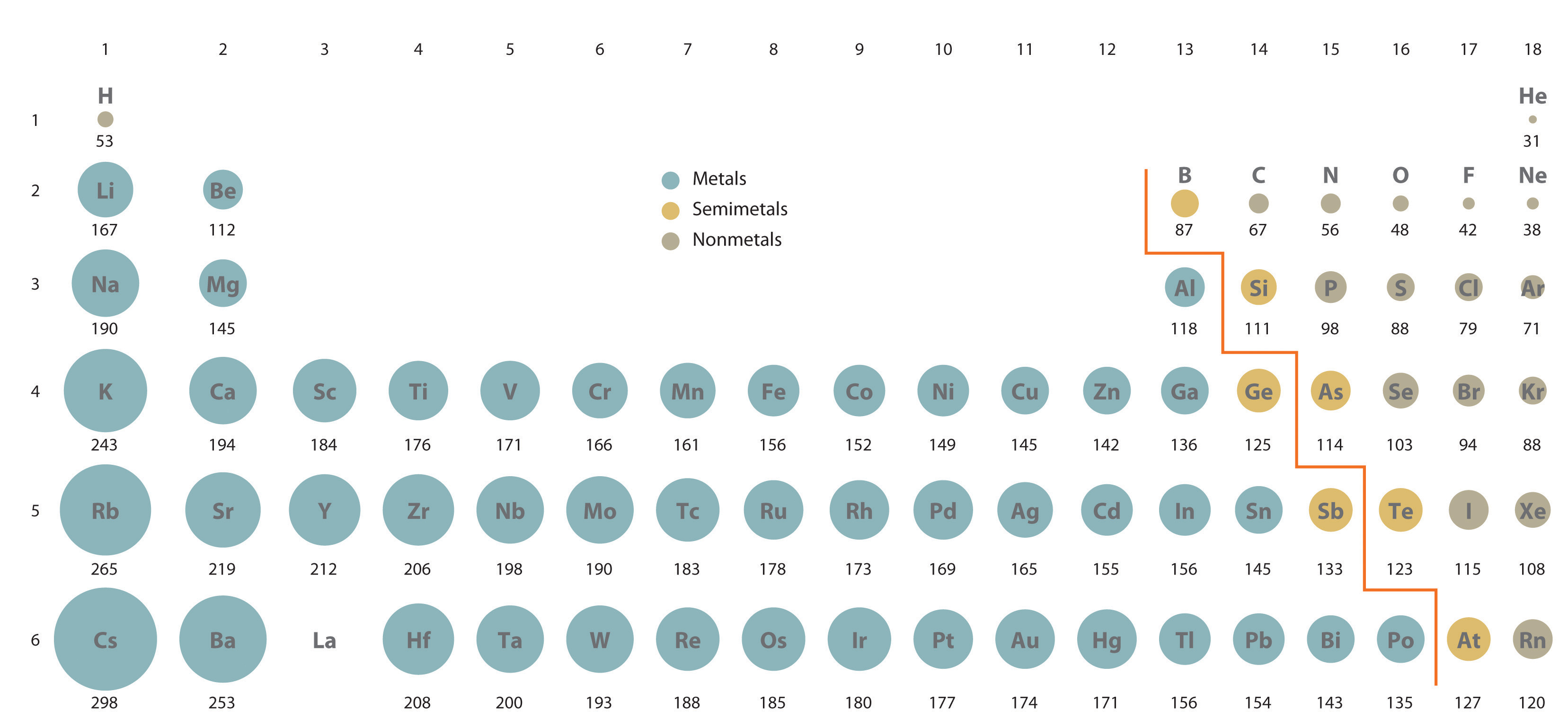
We know that with respect to atoms, atomic size decreases from left to right across a period, a row of the periodic table as we face the table, and increases down a group, a column of the periodic table. Ionic radius (which ion is smaller?) if playback doesn�t begin shortly, try restarting your device. Well, nothing succeeds like measurement.
 Source: transtutors.com
Source: transtutors.com
Which of the following elements has the smallest atomic radius? It may be defined as the effective distance from the nucleus of the ion to the point to which it has an influence in the ionic bond. A) argon b) neon c) helium d) krypton 6 using shorthand nation, the ground sae election conualin to 7.
Up to 40 properties (chemical & physical); Which element has a smaller ionic radius than magnesium mg brainly? Well, nothing succeeds like measurement.
 Source: youtube.com
Source: youtube.com
Thus, helium is the smallest element, and francium is the largest. Helium has the smallest atomic radius. The ionic radius of ni2+ is minimum.
 Source: study.com
Source: study.com
This site offers comprehensive information for each element including: The smallest radius of b 3 + is due to the fact that the high nuclear charge of +3 would be pulling the electron density towards itself more strongly than a +2 or +1. A detailed explanation is given below:
 Source: bigbangpokemon.com
Source: bigbangpokemon.com
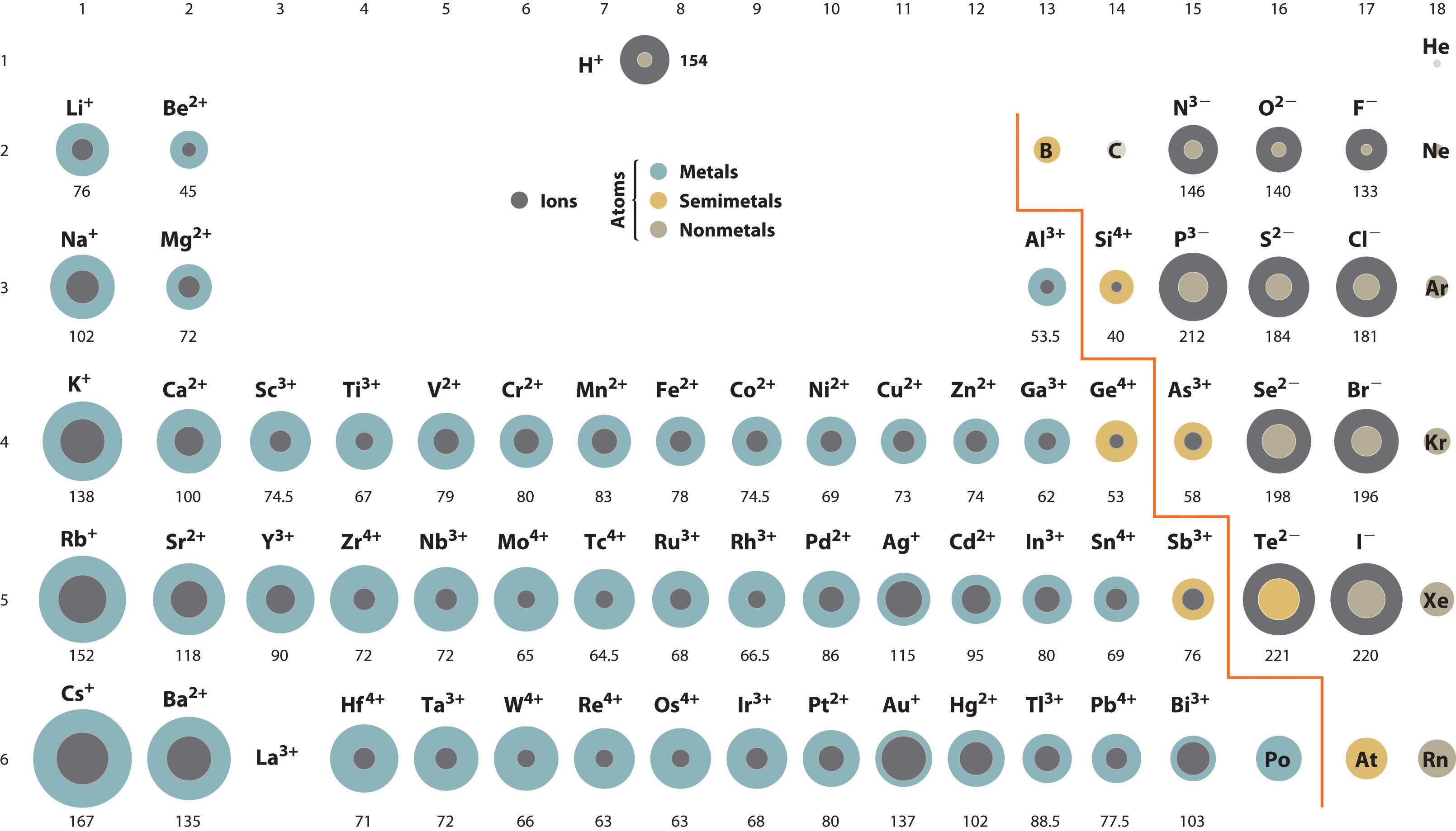
The ionic radius is half the distance between two gas atoms that are just touching each other. Which element’s ionic radius is smaller? As can be seen in the figures below, the atomic radius increases from top to bottom in a group, and decreases from left to right across a period.
 Source: chegg.com
Source: chegg.com
The smallest radius of b 3 + is due to the fact that the high nuclear charge of +3 would be pulling the electron density towards itself more strongly than a +2 or +1. In the ions given about, it can be deducted that they all have the same number of electrons and are said to be isoelectronic. Which element’s ionic radius is smaller?
 Source: scientifictutor.org
Source: scientifictutor.org
The ionic radius of ni2+ is minimum. Ca2+ would have the smallest ionic radius because calcium has a positive charge, and because this ion is a cation, cations will have the smallest radius. The ionic radius of ni2+ is minimum.
 Source: chem.libretexts.org
Source: chem.libretexts.org
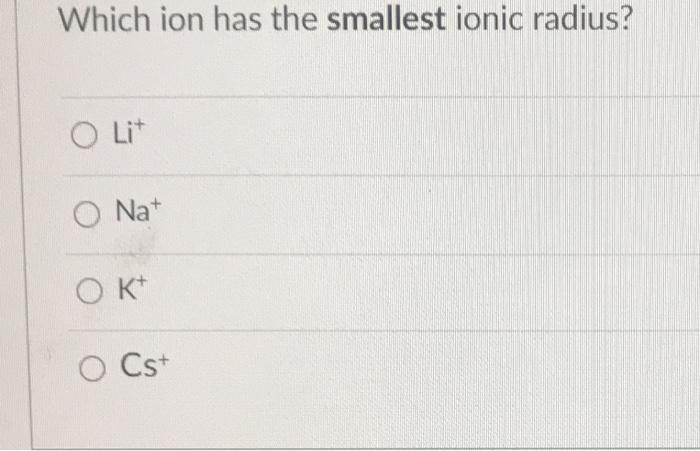
Which is bigger li or mg? Over 4,400 nuclide decay modes; Ionic radius is the distance between the nucleus and the electrons in the outermost shell of an ion.
 Source: studylib.net
Source: studylib.net
The ionic radius of ni2+ is minimum. Correct option is c) l i +, b e 2 + a n d b 3 + are isoelectronic species. The best examples of elements where the ionic radius is meaningful are the group 1, 2 metals, oxide ion and the lighter halogens.
 Source: doubtnut.com
Source: doubtnut.com
In addition chemistry and technical terms are linked to their definitions in the site�s chemistry and. In the case of metals, atomic radius is called metallic radius. As the protons are added, electrons are attracted with a greater strength towards the core, making the radius of mg smaller.
 Source: en.wikipedia.org
Source: en.wikipedia.org
Correct option is c) l i +, b e 2 + a n d b 3 + are isoelectronic species. While comparing the ionic radius, we should consider all the points. Which of the following alkali metal ions has the lowest ionic mobility in aqueous solutions?
 Source: actingcolleges.org
Source: actingcolleges.org
As can be seen in the figures below, the atomic radius increases from top to bottom in a group, and decreases from left to right across a period. So, the correct answer is option c. Consequently, the ion with the greatest nuclear charge (al3+) is the smallest, and the ion with the smallest nuclear charge (n3−) is the largest….ionic radii and isoelectronic series.
 Source: slideplayer.com
Source: slideplayer.com
A detailed explanation is given below: As the protons are added, electrons are attracted with a greater strength towards the core, making the radius of mg smaller. So, in the end, we can conclude that nickel ions have a minimum radius.

Which ion has the largest radius? Ionic radius is the distance between the nucleus and the electrons in the outermost shell of an ion. So, in the end, we can conclude that nickel ions have a minimum radius.
 Source: chegg.com
Source: chegg.com
Up to 40 properties (chemical & physical); The cation , which is an ion with a positive charge, by. Which of the following elements has the smallest atomic radius?
Also Read :