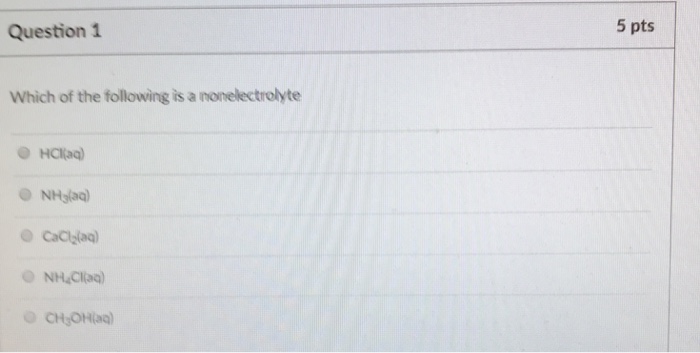
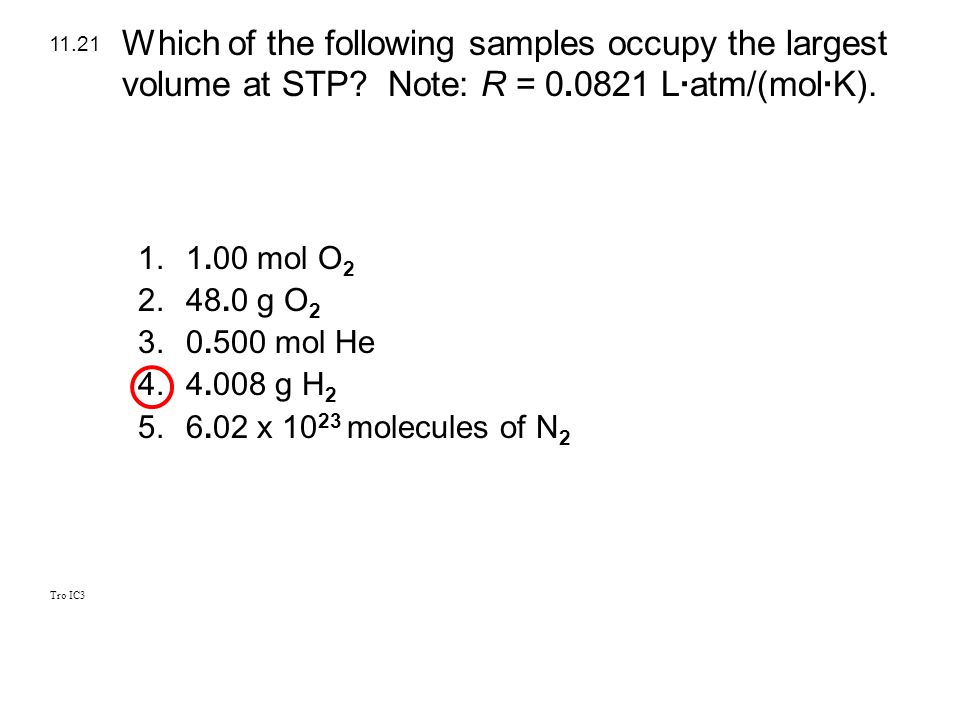
(2) co2 (3) cl2 (4) so2 3. The molar volume is the same (22.4 l) for every ideal gas.
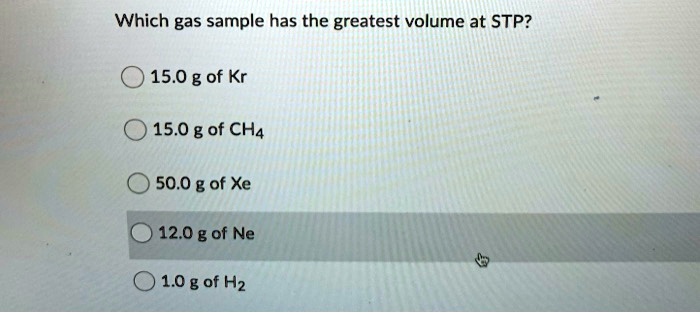
Which Gas Sample Has The Greatest Volume At Stp. Part a assuming ideal behavior, which of these gas samples has the greatest volume at stp? The molar volume of a gas expresses the volume occupied by 1 mole of that respective gas under certain temperature and pressure conditions. We are asked which of the gas samples has the greatest volume at stp. This means the lowest molar mass will have the highest volume.

Related Post Solved Pressure At Stp 2) Which Of The Following Samples | Chegg.com :
2 get other questions on the subject: Which gas sample has the greatest volume at stp? None of the above (all have the same kinetic energy) 1 see answer Room conditions refer to the temperature of 25°c and the pressure of 1 atmosphere.
Other questions on the subject:
The correct statement is (c). Assuming ideal behavior, which of these gas samples has the greatest volume at stp? None of the above (all have the same kinetic energy) 1 see answer Tap card to see definition 👆. At stp, which gas would most likely behave as an ideal gas? Which gas has the greatest kinetic energy at stp?
 Source: numerade.com
Source: numerade.com
Requirements change every four years requirements change every year august 2016 requirements change every other year. We are asked which of the gas samples has the greatest volume at stp. So here helium, hey insta greater has greater volume.
 Source: chegg.com
Source: chegg.com
Yeah, since it is the lowest molecule er moss. 2 get other questions on the subject: The correct statement is (c).
 Source: slideplayer.com
Source: slideplayer.com
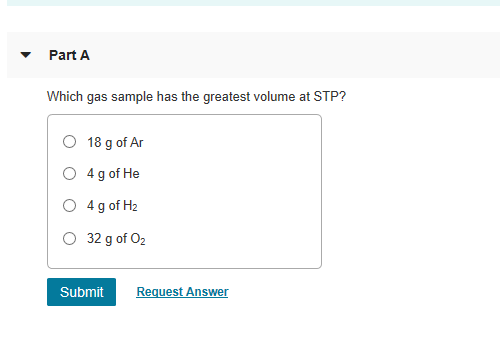
So as here 10 grandma helium into one more layer of helium, 4.0 program. O 1g of he 0 1 g of xe o 1g of f2 submit request answer provide feedback. When will division i requirements change?
 Source: numerade.com
Source: numerade.com
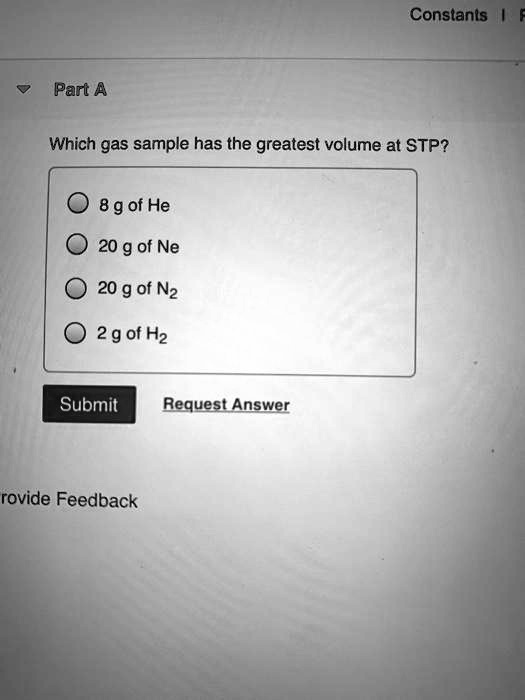
This means that the #moles of gas is proportional to the volume of gas at constant t and p. Here we are asked for the gas that has the greatest volume but the problem here is that we are only given the mass values. Hence, from the above calculations, we see that option d has the largest amount of moles and hence, will have the largest amount of particles.
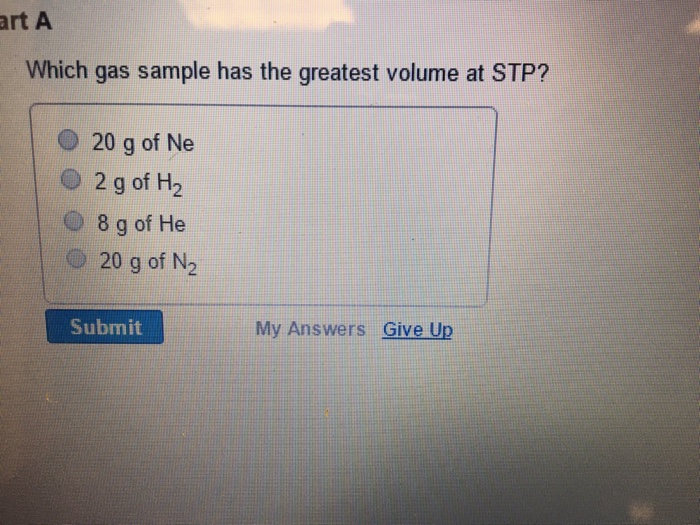
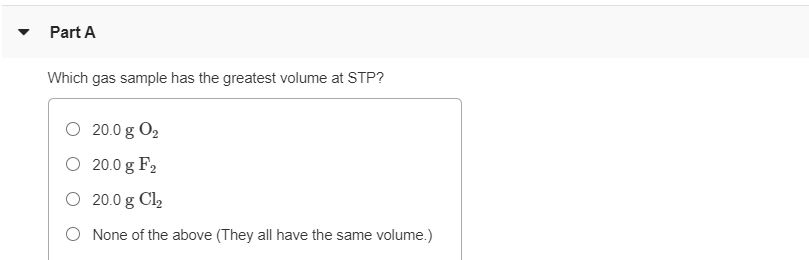
Which gas sample has the greatest volume at stp? Check out a sample q&a here. (1) o2 (2) h2 (3) nh3 (4) hcl 4.
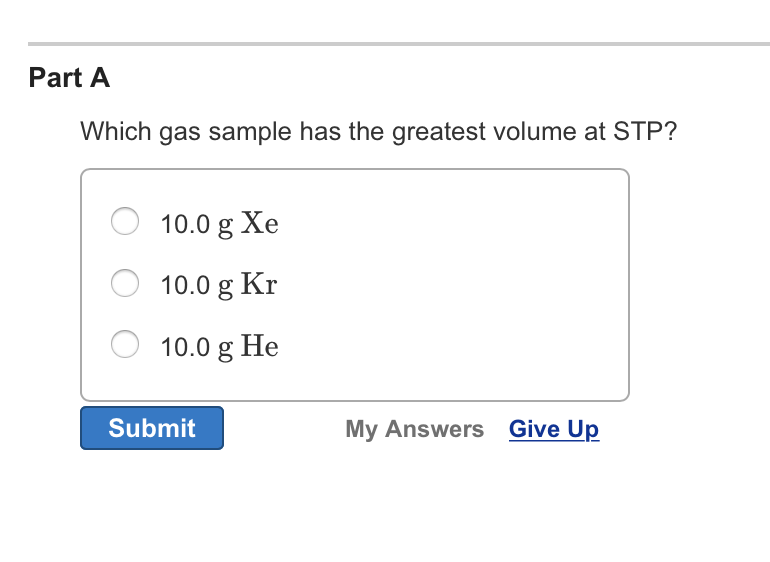 Source: chegg.com
Source: chegg.com
According to avogadro, equal volumes of gas at the same temperature and pressure will contain the same number of moles of gas. This means that one mole. We review their content and use your feedback to keep the quality high.
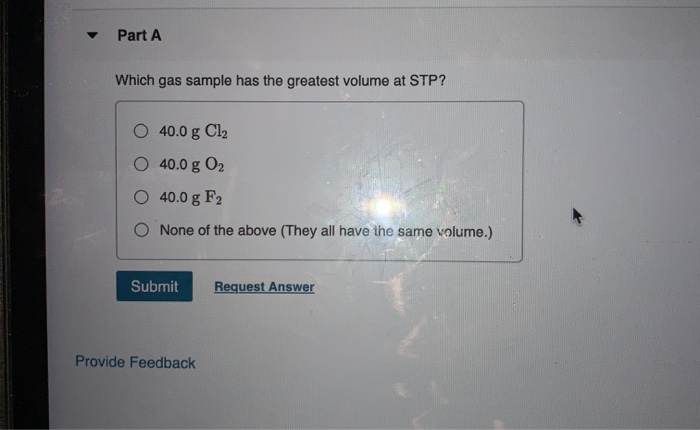 Source: chegg.com
Source: chegg.com
We are asked which of the gas samples has the greatest volume at stp. One cup of milk contains 299 mg of calcium and one cup of juice contains 261 mg of calcium. Assuming ideal behavior, which of the following gas samples will have the greatest volume?
 Source: clutchprep.com
Source: clutchprep.com
One cup of milk contains 299 mg of calcium and one cup of juice contains 261 mg of calcium. The condition is also stated to be at stp (standard temperature and pressure). Which gas sample has the greatest volume at stp?

What is the pressure (in atm) when the volume of the sample is decreased to 223 ml ? The given masses are the same. Other questions on the subject:
 Source: clutchprep.com
Source: clutchprep.com
(1) o2 (2) h2 (3) nh3 (4) hcl 4. This means the lowest molar mass will have the highest volume. The gas which has the largest number of moles, will have the largest number of particles.
 Source: numerade.com
Source: numerade.com
(assume constant temperature and constant number of moles of gas.) click card to see definition 👆. So, if you convert the mass of each gas to moles (#moles = m. What is the volume of the gas at stp.
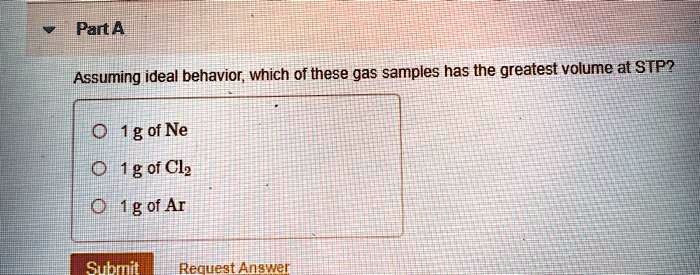 Source: numerade.com
Source: numerade.com
Assuming ideal behavior, which of these gas samples has the greatest volume at stp?. This gives us a clue that we can use 1 mole = 22.4 l. We review their content and use your feedback to keep the quality high.
 Source: numerade.com
Source: numerade.com
Which gas sample has the greatest volume at. Hence, from the above calculations, we see that option d has the largest amount of moles and hence, will have the largest amount of particles. A gas sample has an initial pressure of 578 mmhg and an initial volume of 0.512 l.
 Source: chegg.com
Source: chegg.com
The correct statement is (c). This means that the #moles of gas is proportional to the volume of gas at constant t and p. According to avogadro, equal volumes of gas at the same temperature and pressure will contain the same number of moles of gas.
 Source: clutchprep.com
Source: clutchprep.com
Stp refers to standard temperature of 0°c and pressure of 1 atmosphere. See the answer see the answer see the answer done loading. This gives us a clue that we can use 1 mole = 22.4 l.
 Source: chegg.com
Source: chegg.com
A sample of co2 occupies 6.00l at 25c and 700. (1) o2 (2) h2 (3) nh3 (4) hcl 4. Which gas sample has the greatest volume at stp?
 Source: chegg.com
Source: chegg.com
We are asked which of the gas samples has the greatest volume at stp. The molar volume of a gas expresses the volume occupied by 1 mole of that respective gas under certain temperature and pressure conditions. Hence, from the above calculations, we see that option d has the largest amount of moles and hence, will have the largest amount of particles.
 Source: clutchprep.com
Source: clutchprep.com
We are asked which of the gas samples has the greatest volume at stp. Which gas sample has the greatest volume at stp? Requirements change every four years requirements change every year august 2016 requirements change every other year.
 Source: slideplayer.com
Source: slideplayer.com
Which gas has properties that are most similar to those of an ideal gas? The gas which has the largest number of moles, will have the largest number of particles. So as here 10 grandma helium into one more layer of helium, 4.0 program.
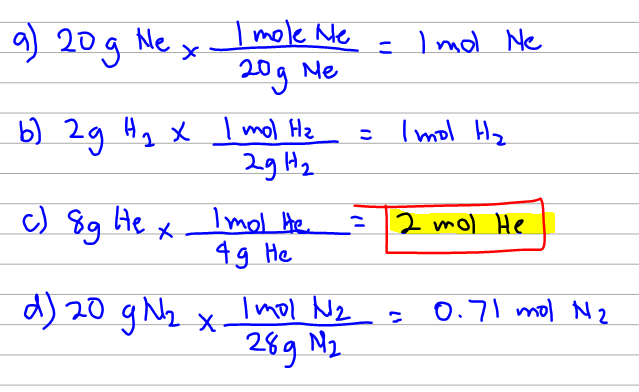 Source: clutchprep.com
Source: clutchprep.com
Want to see this answer and more? Assuming ideal behavior, which of the following gas samples will have the greatest volume? This means that the #moles of gas is proportional to the volume of gas at constant t and p.
Also Read :