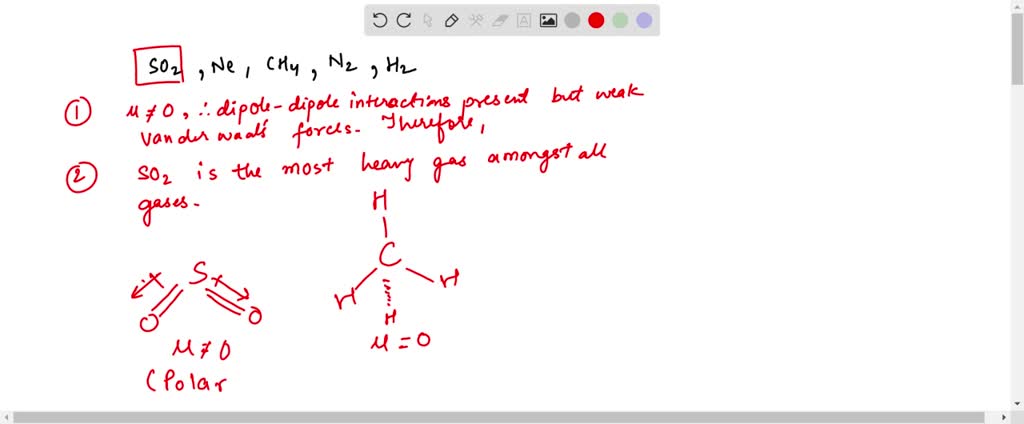
Also, this same gas has higher mp and bp than the other gases and significantly closer to room temperature. All gases are real gases.
Which Gas Deviates Most From Ideal Behavior. What gases behave most like an ideal gas? Lower density gases are more likely to behave as ideal gases. The gas in sample 2 would deviate more from ideal behavior because the xe atoms are closer together, leading to an increase in intermolecular attractions. Such gases are, therefore, known as �real gases�.

Related Post Ktufsd.org :
Learn vocabulary, terms, and more with flashcards, games, and other study tools. (i) low pressure (ii) high pressure (iii) low temperature (iv) high temperature; B mass c velocity d attractions. Such gases are, therefore, known as ‘real gases’.
The real gas that acts most like an ideal gas is helium.
For a gas to be “ideal” there are four governing. Deviations from ideal gas law behavior can be described by the van der waals equation, which includes empirical constants to correct for the actual volume of the gaseous molecules and quantify the reduction in pressure due to intermolecular attractive forces. Lower density gases are more likely to behave as ideal gases. As all gases have the same volume, the gas with the greater molar mass will have the higher density. Such gases are, therefore, known as �real gases�. Gases like nh3, hcl, h2o, co2 are large and polar.
 Source: numerade.com
Source: numerade.com
At high pressure and low temperature, a gas deviates the most from its ideal behaviour. Learn vocabulary, terms, and more with flashcards, games, and other study tools. As the temperature increases, the deviation from that of the ideal gas behaviour decreases and the ideal gas law can be used to predict.

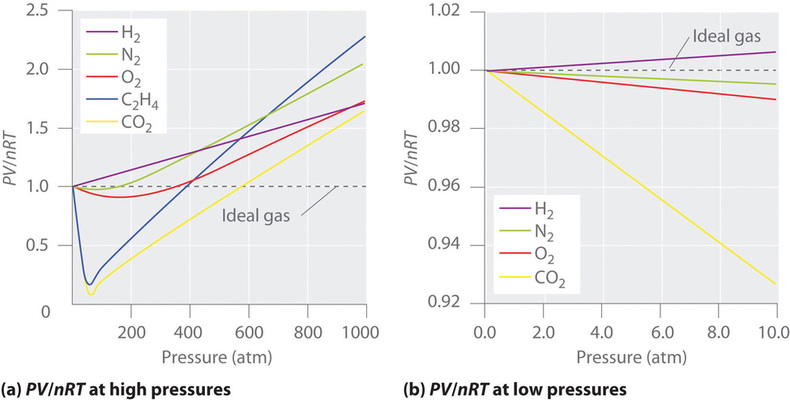
They show deviation from ideal behavior. For gases such as hydrogen, oxygen, nitrogen, helium, or neon, deviations from the ideal gas law are less than 0.1 percent at room temperature and atmospheric pressure. The real gas that acts most like an ideal gas is helium.
 Source: slideplayer.com
Source: slideplayer.com
B mass c velocity d attractions. Hence, at high pressure and low temperature, a real gas deviates most from ideal behaviour. All gases are real gases.

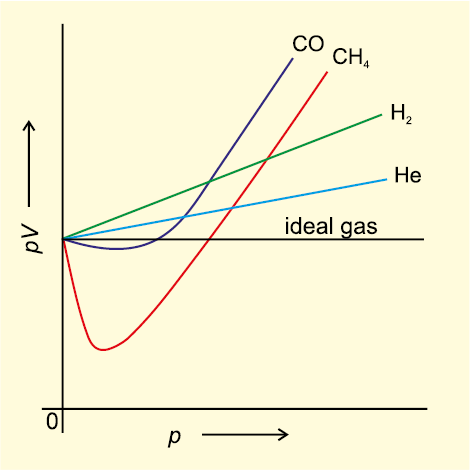
Are the ones likely to deviate most from ideal behavior. The gases are found to obey the gas laws fairly well when the pressure is low or the temperature is high. Gases most closely approximate ideal gas behavior at high temperatures and low pressures.
 Source: neetprep.com
Source: neetprep.com
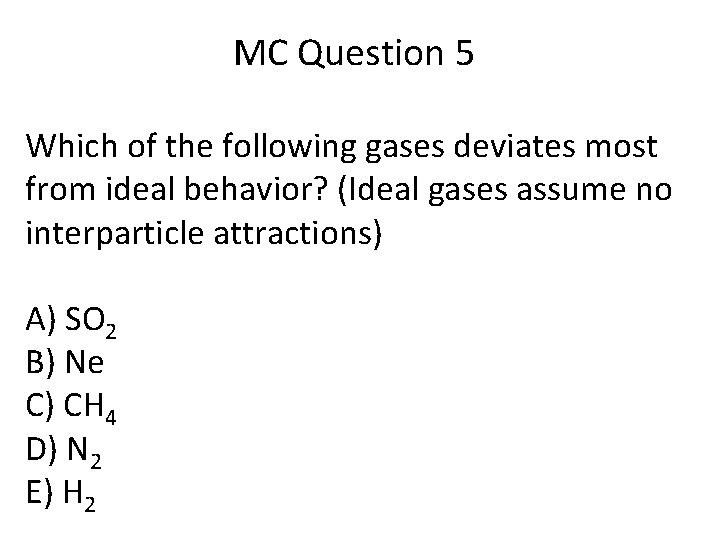
Therefore gases with large polar molecules like ethers, alcohols, aldehydes, ketones etc. Which of the following gases deviates most from ideal behavior? Which gas would behave least ideally?
 Source: slidetodoc.com
Source: slidetodoc.com
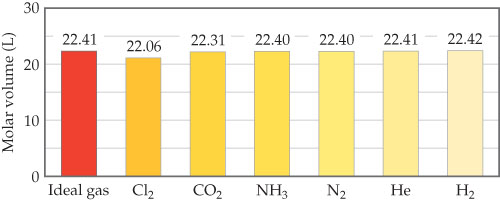
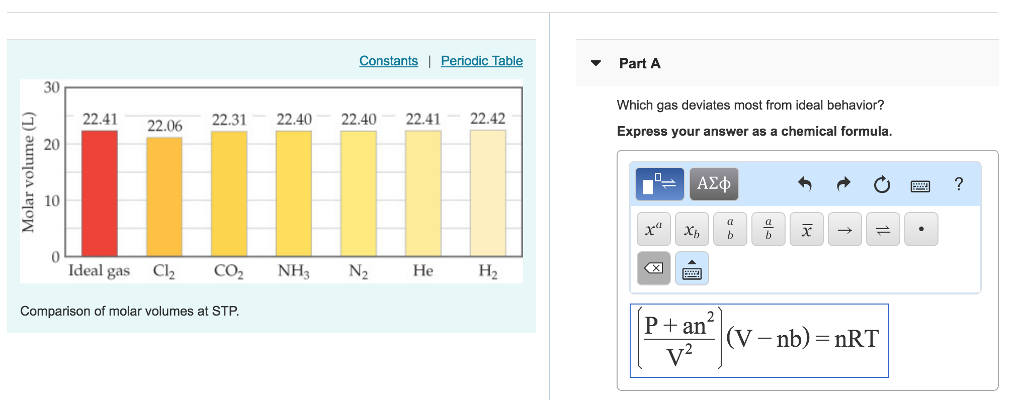
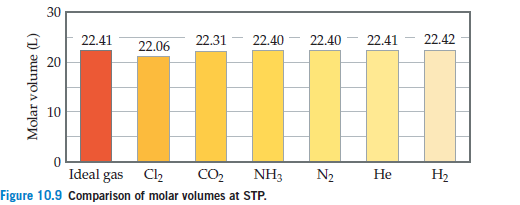
Start studying chem test review. 22.41 226 22.31 22.40 22.40 22.41 22.42 20 express your answer as a chemical formula 10 2 (l 0 ideal gas c12 co2 nh3 n2 he h2 comparison of molar volumes at stp. Also, what is real gas equation?
 Source: youtube.com
Source: youtube.com
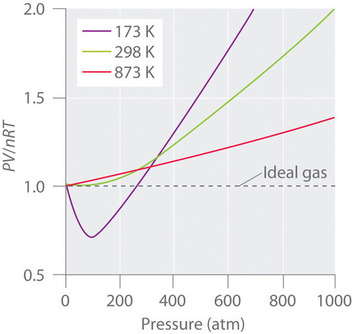
Are the ones likely to deviate most from ideal behavior. Learn vocabulary, terms, and more with flashcards, games, and other study tools. When the actual gas volume is greater than the volume predicted by the ideal gas law, the lanation lies in the fact that the ideal gas law does not include a factor for molecularexp a volume.
 Source: chem.libretexts.org
Source: chem.libretexts.org
Most from the ideal behaviour? Which of the following gases deviates most from ideal behavior? Such gases are, therefore, known as ‘real gases’.
 Source: clutchprep.com
Source: clutchprep.com
Gases are most ideal at high temperature and low pressure. For a gas to be “ideal” there are four governing. Which of the following gases deviates most from ideal behavior?

Which sample deviates most from. Such gases are, therefore, known as ‘real gases’. Therefore gases with large polar molecules like ethers, alcohols, aldehydes, ketones etc.

The gases are found to obey the gas laws fairly well when the pressure is low or the temperature is high. Hence, the concept of ideal gas is only theoretical or hypothetical. Real gases deviate from ideal behaviour because their particles (atoms for inert gases or molecules) occupy some finite space and do exert interactive forces among them.
 Source: slideplayer.com
Source: slideplayer.com
So when deciding how far a gas deviates from an ideal gas we can look at how strong the attraction is between its gas molecules. So when deciding how far a gas deviates from an ideal gas we can look at how strong the attraction is between its gas molecules. Start studying chem test review.
 Source: clutchprep.com
Source: clutchprep.com
Which of the following gases deviates most from ideal behavior? B mass c velocity d attractions. Another factor is that helium, like other noble gases, has a completely filled outer electron shell.
 Source: chemed.chem.purdue.edu
Source: chemed.chem.purdue.edu
At high pressure and low temperature, a gas deviates the most from its ideal behaviour. Hence, at high pressure and low temperature, a real gas deviates most ideal behaviour. Part a 30 which gas deviates most from ideal behavior?

Which gas would behave least ideally? Hence, the concept of ideal gas is only theoretical or hypothetical. B mass c velocity d attractions.
 Source: chegg.com
Source: chegg.com
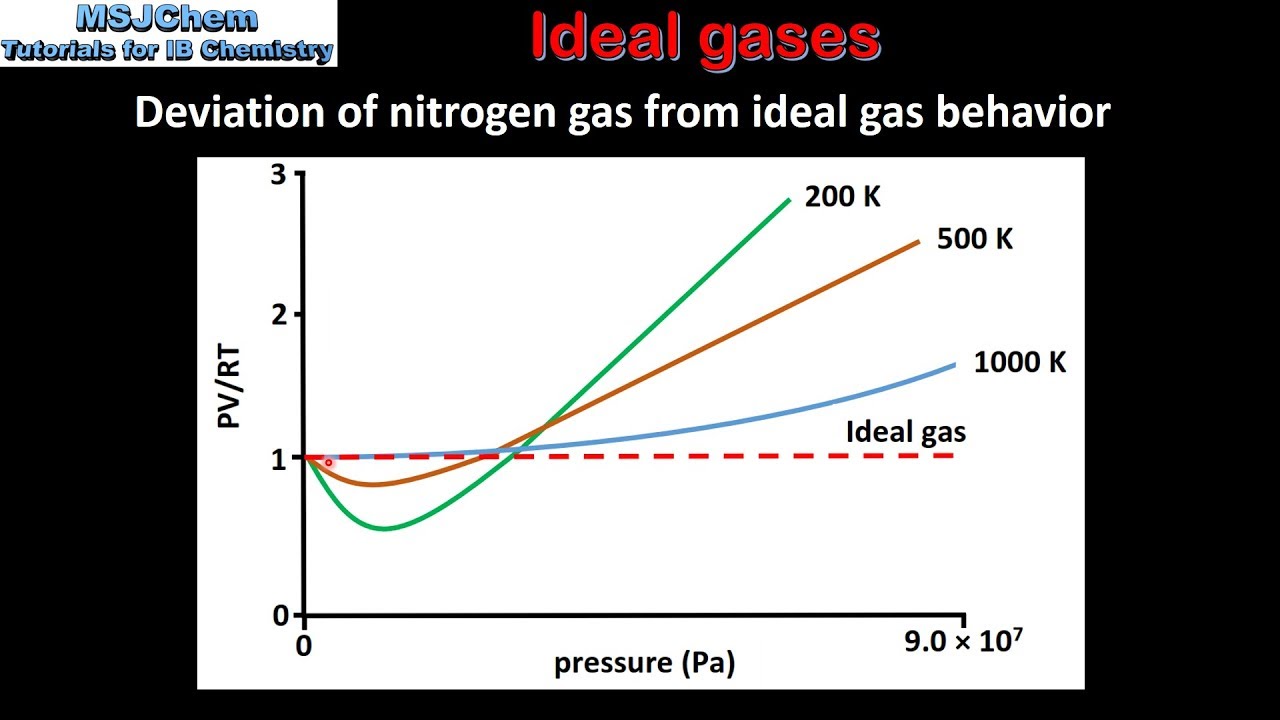
Hence, at high pressure and low temperature, a real gas deviates most ideal behaviour. Nitrogen gas that has been cooled to 77 k has turned to a liquid and must be stored in a vacuum insulated container to prevent it from rapidly vaporizing. For gases such as hydrogen, oxygen, nitrogen, helium, or neon, deviations from the ideal gas law are less than 0.1 percent at room temperature and atmospheric pressure.
 Source: chem.libretexts.org
Source: chem.libretexts.org
Lower density gases are more likely to behave as ideal gases. What gases behave most like an ideal gas? Gases act most like ideal gases when the molecules have low mass (small volume), are not polar, and are at high temperature and low pressure.
 Source: chegg.com
Source: chegg.com
If you like this answer, please upvote as a token of your appreciation. At high pressure and low temperature, a gas deviates the most from its ideal behaviour. Real gases deviate from ideal behaviour because their particles (atoms for inert gases or molecules) occupy some finite space and do exert interactive forces among them.

Also gases are more likely to be ideal at temperatures well above the liquefying point. Hence, the concept of ideal gas is only theoretical or hypothetical. What gases behave most like an ideal gas?
 Source: bartleby.com
Source: bartleby.com
What makes a gas deviate from ideal behavior? 22.41 226 22.31 22.40 22.40 22.41 22.42 20 express your answer as a chemical formula 10 2 (l 0 ideal gas c12 co2 nh3 n2 he h2 comparison of molar volumes at stp. Option (ii) and (iii) are the answers.
Also Read :