1.3) the hydrocarbon c 4 h 8 was burnt in air. Hydrocarbon + o 2 → co 2 + h 2 o.
Which Equation Represents A Combustion Reaction. The general form of a combustion reaction can be represented by the reaction between a hydrocarbon and oxygen, which yields carbon dioxide and water: If you are having trouble with chemistry, organic, physics, calculus, or statistics, we got your back! A hydrocarbon as the fuel. C 6 h 12 o 6 (s) + 6o 2 (g) → 6co 2 (g) + 6h 2 o (g) which is correct for this reaction?
 Combustion Reaction | Ck-12 Foundation From ck12.org
Combustion Reaction | Ck-12 Foundation From ck12.org
Related Post Combustion Reaction | Ck-12 Foundation :
Hydrocarbon + o 2 → co 2 + h 2 o. The chemical equation, kclo3 → kcl + o2, is an example of which type of reaction? These reactions are the opposite of endothermic reactions and can be expressed in a chemical equation as follows: An example of a hydrocarbon is methane.
Hexane was burnt in a crucible and incomplete combustion occurred.
Combustion reactions can be stopped by. The general form of a combustion reaction can be represented by the reaction between a hydrocarbon and oxygen, which yields carbon dioxide and water: Let us help you simplify your studying. Of the balanced chemical equations below, which one is combustion reaction? 2ch4 + 2o2 → co2 + 2h2o c. A hydrocarbon as the fuel.
 Source: slideshare.net
Source: slideshare.net
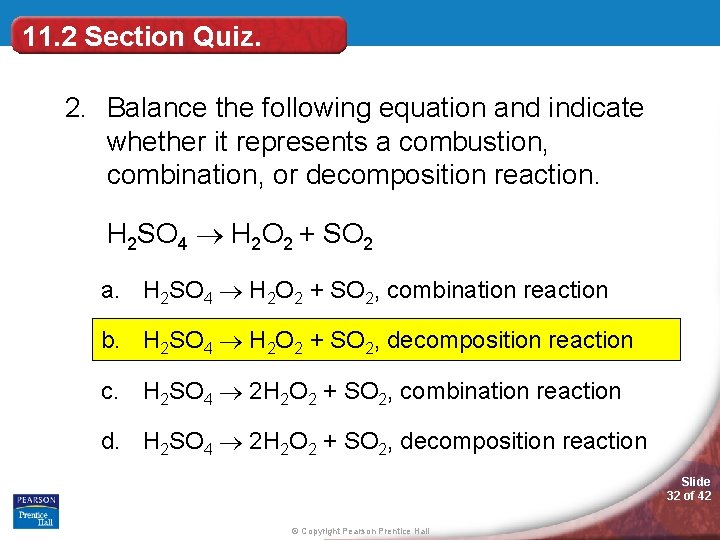
2 ch 4 + 4 o 2 → 2 co 2 + 4 h 2o b. In this reaction, hydrogen react with the chlorine to form a single product as hydrogen chloride. Terms in this set (9) combustion reaction.
 Source: nagwa.com
Source: nagwa.com
Hexane was burnt in a crucible and incomplete combustion occurred. The image shows the combustion of methane. Cao + h 2o → ca(oh) 2 c.

An exothermic reaction is a chemical reaction that involves the release of energy in the form of heat or light. If not enough oxygen is present for complete combustion, incomplete combustion occurs. Let us help you simplify your studying.
 Source: ck12.org
Source: ck12.org
An exothermic reaction is a chemical reaction that involves the release of energy in the form of heat or light. Terms in this set (9) combustion reaction. Terms in this set (40) calcium reacts with oxygen to form calcium oxide.
 Source: slideplayer.com
Source: slideplayer.com
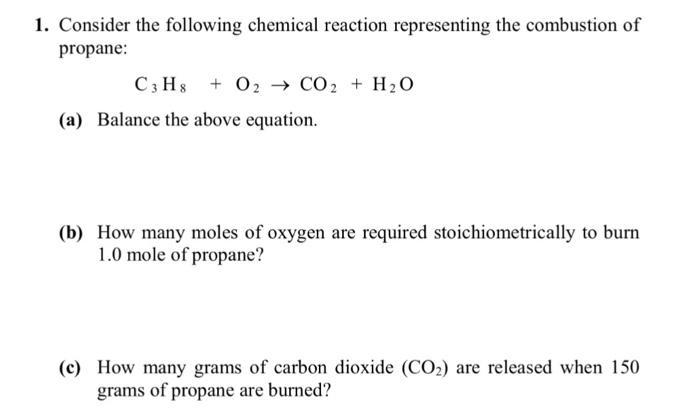
If you are having trouble with chemistry, organic, physics, calculus, or statistics, we got your back! 2 ch 4 + 4 o 2 → 2 co 2 + 4 h 2o b. Which equation represents incomplete combustion?
 Source: brainly.com
Source: brainly.com
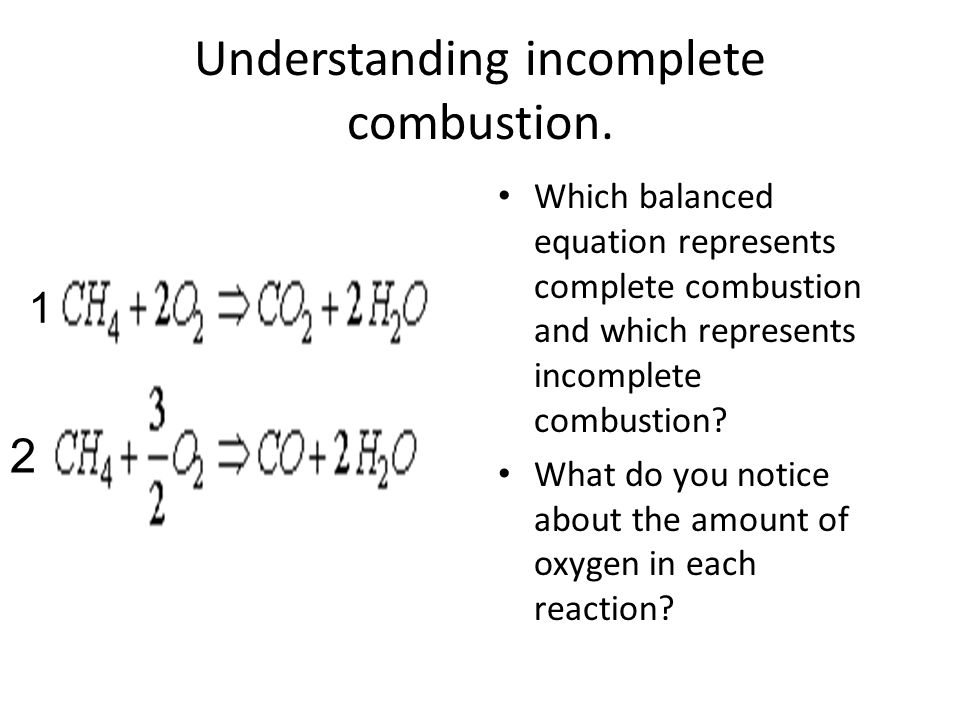
Which equation represents the lattice enthalpy of magnesium sulfide? It�s the synthesis reaction also called combination reaction or composition reaction. 1.3) the hydrocarbon c 4 h 8 was burnt in air.
Let us help you simplify your studying. The chemical equation, kclo3 → kcl + o2, is an example of which type of reaction? The general equation for a complete combustion reaction is:
 Source: brainly.com
Source: brainly.com
Choose the three numbers that, in the correct order, balance the following incomplete combustion reaction: 1.2) table 1 shows the boiling point, flammability and viscosity of c 18 h 38 compared with the other hydrocarbons shown in the equation. Our videos will help you understand concepts, solve your homework, and do great on your exams.
 Source: slideplayer.com
Source: slideplayer.com
Oxyacetylene is the hottest burning common fuel gas. The chemical equation for this reaction is ca+o2→cao. In this reaction, hydrogen react with the chlorine to form a single product as hydrogen chloride.
 Source: web.fscj.edu
Source: web.fscj.edu
The combustion of acetylene gas is represented by this equation: A combustion reaction is a reaction that burns. Ch4 + o2 → 2co2 + 2 h2o e.
 Source: slidetodoc.com
Source: slidetodoc.com
4 co2 + 2 h2o, two molecules of acetylene combine with five compounds of two bonded oxygen atoms to form four. Learn the combustion reaction definition and what happens in a combustion reaction. An example of a hydrocarbon is methane.
![Solved] The Following Equation Represents The Complete Combustion Of Butane, C4H10. C4H10(G) + 6.5O2(G) 4Co2(G) + 5H2O(G) (A) Using Known Enthalpies… | Course Hero](https://www.coursehero.com/qa/attachment/15957426/ “Solved] The Following Equation Represents The Complete Combustion Of Butane, C4H10. C4H10(G) + 6.5O2(G) 4Co2(G) + 5H2O(G) (A) Using Known Enthalpies… | Course Hero”) Source: coursehero.com
Choose the three numbers that, in the correct order, balance the following incomplete combustion reaction: An exothermic reaction is a chemical reaction that involves the release of energy in the form of heat or light. The following equation represents a combustion reaction of propane, c3h8(g) when the oxygen supply is limited.
 Source: study.com
Source: study.com
A combustion reaction is a reaction that burns. A combustion reaction is a reaction that burns. It�s the synthesis reaction also called combination reaction or composition reaction.
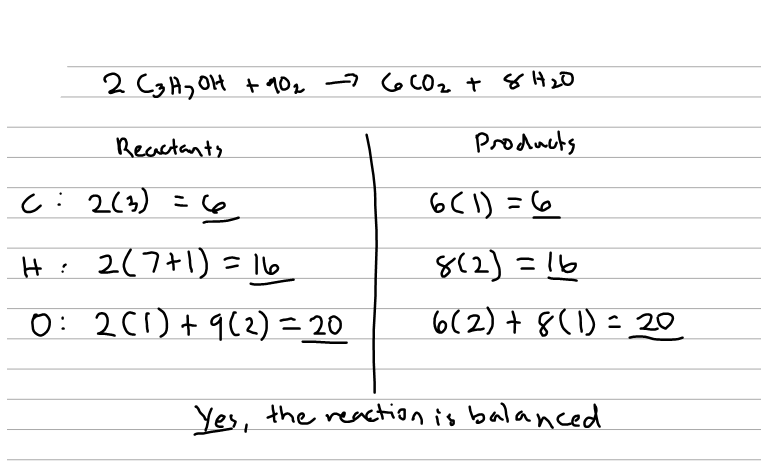 Source: clutchprep.com
Source: clutchprep.com
The chemical equation, kclo3 → kcl + o2, is an example of which type of reaction? 2 n 2 + 3 h 2 → 2 nh 3 d. Alcl 3 + naoh → x + nacl a.

In this reaction, hydrogen react with the chlorine to form a single product as hydrogen chloride. Combustion reactions can be stopped by. Which equation represents the standard enthalpy of formation for ethanol c2h5oh?
 Source: numerade.com
Source: numerade.com
Understand the combustion reaction equation and examples of combustion in everyday life. If not enough oxygen is present for complete combustion, incomplete combustion occurs. If you are having trouble with chemistry, organic, physics, calculus, or statistics, we got your back!
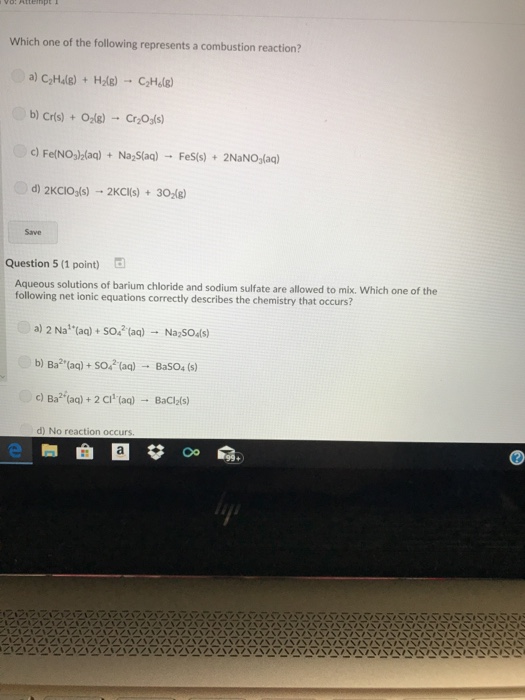
Understand the combustion reaction equation and examples of combustion in everyday life. Ch4 + o2 → co2 +. C 6 h 12 o 6 (s) + 6o 2 (g) → 6co 2 (g) + 6h 2 o (g) which is correct for this reaction?
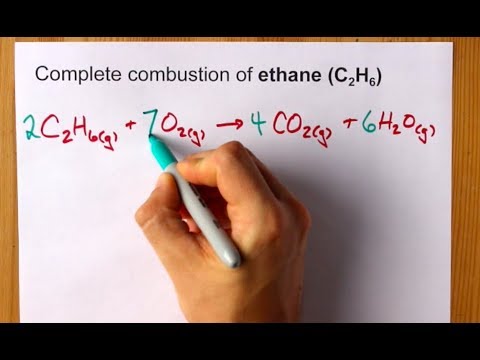 Source: youtube.com
Source: youtube.com
An exothermic reaction is a chemical reaction that involves the release of energy in the form of heat or light. Which equation represents the standard enthalpy of formation for ethanol c2h5oh? The chemical equation for this reaction is ca+o2→cao.
 Source: chegg.com
Source: chegg.com
If you are having trouble with chemistry, organic, physics, calculus, or statistics, we got your back! Terms in this set (9) combustion reaction. What is the product, or what are the products, of this reaction?

1.3) the hydrocarbon c 4 h 8 was burnt in air. Of the balanced chemical equations below, which one is combustion reaction? What are the products of the following combustion… which formula equation represents the burning of… the combustion of pentane, c5h12, occurs via the… what type of reaction takes place when the melting… in which two types of reactions do opposite chemical… consider the following unbalanced equation for the…
Also Read :





