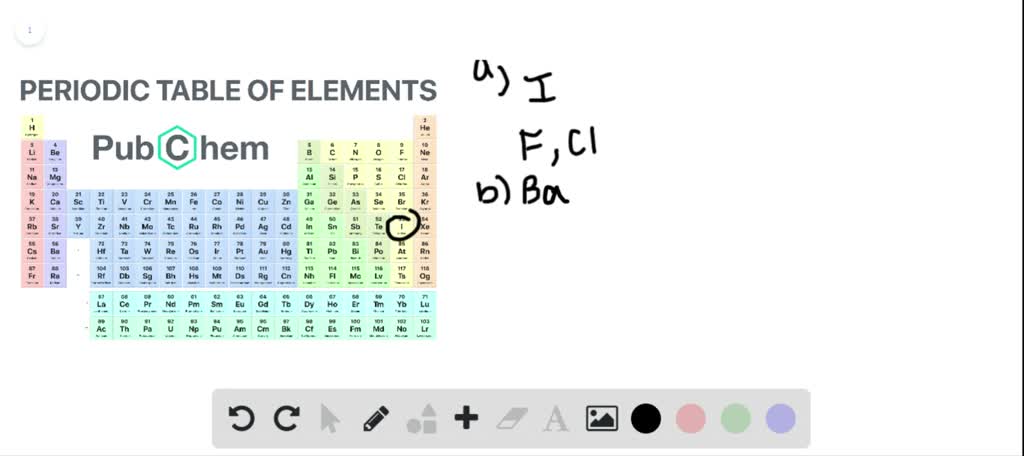
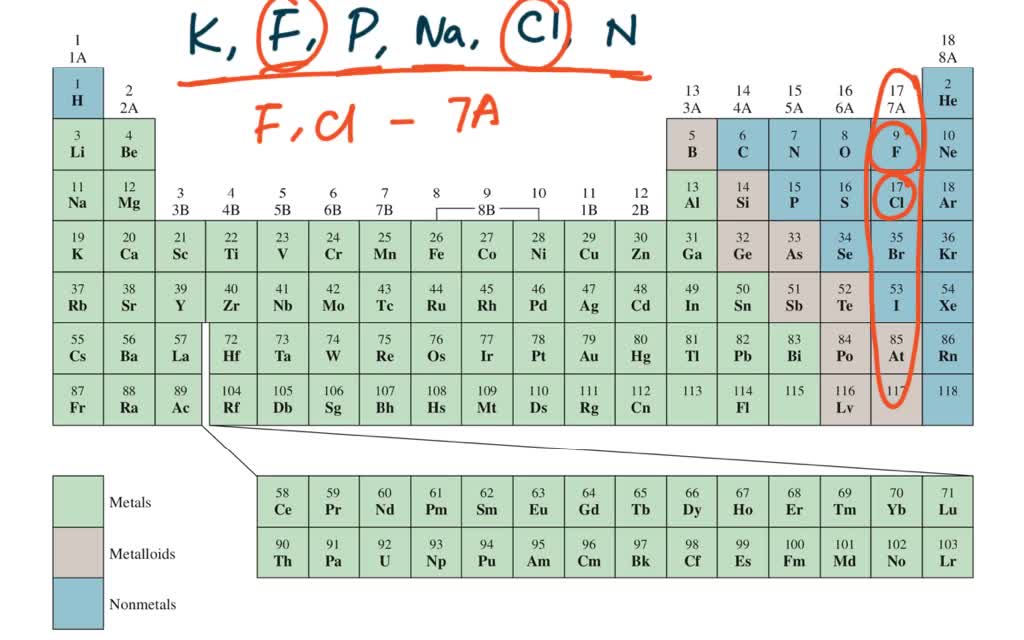
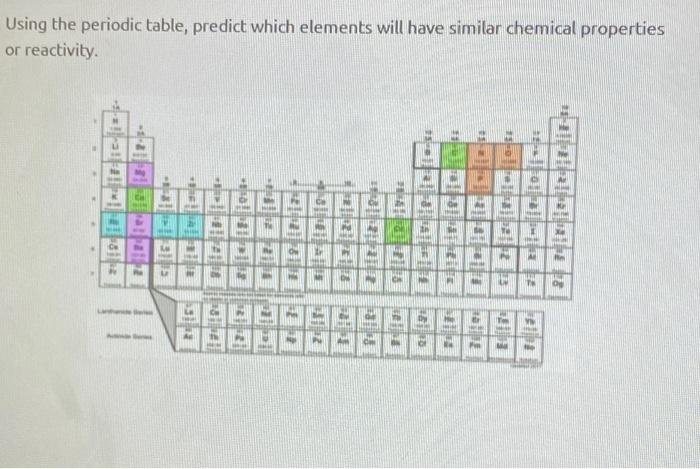
So these three elements have similar chemical properties. A and b, respectively, are :
Which Elements Have Similar Properties. Lithium comes in group 1 of modern periodic table. The elements calcium, strontium and barium were put in one group and family on the basis of their similar properties. For example, when reacting with hydrogen gas, these elements form a polar bent molecule containing two. Elements found in group 2 of the periodic table have similar properties to it.
 Which Of These Sets Of Elements Have Similar Physical And Chemical Properties? - Brainly.com From brainly.com
Which Of These Sets Of Elements Have Similar Physical And Chemical Properties? - Brainly.com From brainly.com
Related Post Which Of These Sets Of Elements Have Similar Physical And Chemical Properties? - Brainly.com :
Calcium is a chemical element with the symbol ca and atomic number 20. What are three elements that have similar chemical properties to lithium? For example, sodium, potassium, lithium, rubidium, and caesium have similar chemical properties. Each element within a group has similar physical or chemical properties because of its atom’s outermost electron shell (most chemical properties are dominated by the orbital location of the outermost electron).
Since the elements having atomic number 13 and 31 have 3 outermost electrons.
Two electron configurations represent elements that would have similar chemical properties are (2) and (4). Magnesium, calcium, strontium, barium and radium have similar properties to beryllium. Elements that are above and below each other in the same vertical column of the periodic table have the same chemical properties because they have the same outer electron shell properties. So, these elements are placed in 3rd group due to which these elements have similar properties. A and b, respectively, are : Oxygen is in group 16/via, which is called the chalcogens, and members of the same group have similar properties.
 Source: courses.lumenlearning.com
Source: courses.lumenlearning.com
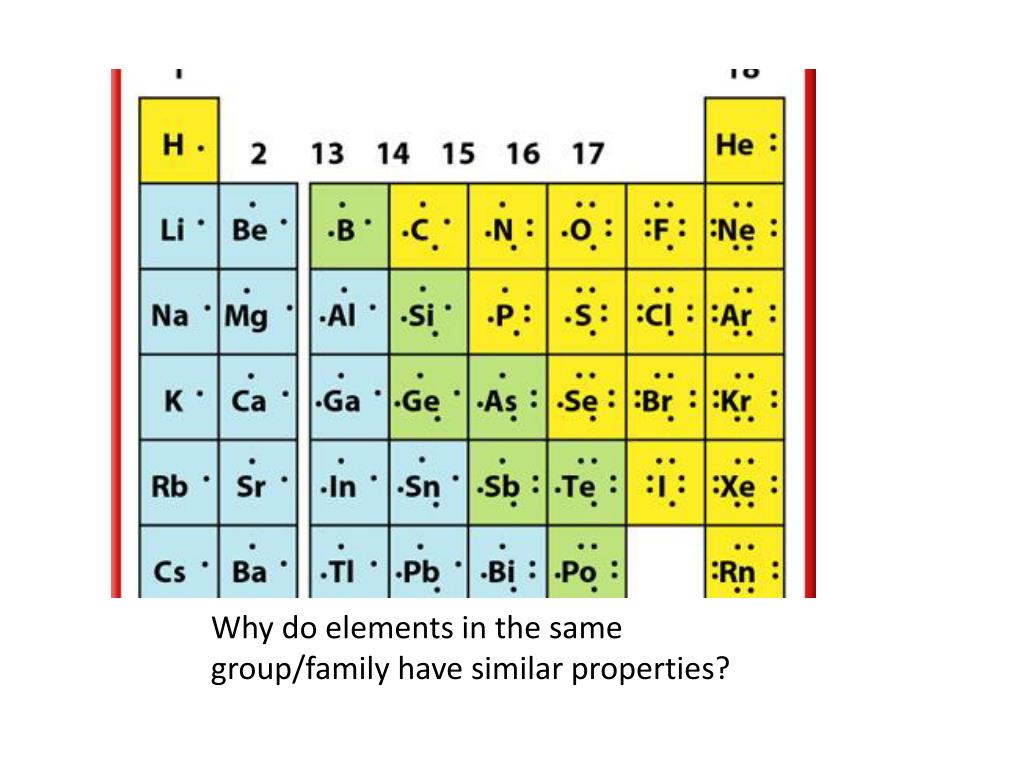
Only choice c refers to elements in the same group. Elements found in group 2 of the periodic table have similar properties to it. Within the same group elements have similar electron configurations because the have the same.
 Source: slideserve.com
Source: slideserve.com
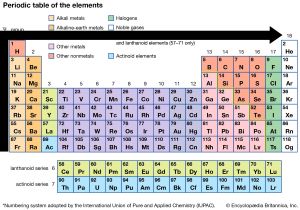
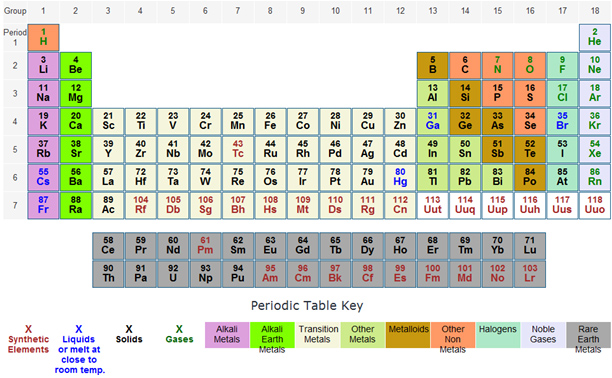
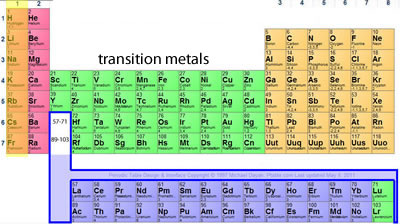
Elements that are above and below each other in the same vertical column of the periodic table have the same chemical properties because they have the same outer electron shell properties. Its physical and chemical properties are most similar to its heavier homologues strontium and barium. When the chemical elements are thus arranged, there is a recurring pattern called the “periodic law” in their properties, in which elements in the same column (group) have similar properties.
 Source: ontrack-media.net
Source: ontrack-media.net
All the elements in group two of the periodic table have the same or similar properties. Since the elements having atomic number 13 and 31 have 3 outermost electrons. For example, sodium, potassium, lithium, rubidium, and caesium have similar chemical properties.
 Source: brighthubengineering.com
Source: brighthubengineering.com
What were those similar properties? Element with atomic number 12 will have similar physical and chemical properties as element with atomic numbers 20 and 38. Since the elements having atomic number 13 and 31 have 3 outermost electrons.
 Source: numerade.com
Source: numerade.com
Lithium comes in group 1 of modern periodic table. Transition metals have similar properties, and some of these properties are different from those of the metals in group 1. The pair of elements that have similar chemical properties are a lithium and magnesium b beryllium and boron c aluminium and magnesium d carbon and nitrogen
 Source: quia.com
Source: quia.com
In general, elements in the same column of the periodic table tend to have similar chemical properties. A and b, respectively, are : Oxygen is in group 16/via, which is called the chalcogens, and members of the same group have similar properties.

What are three elements that have similar chemical properties to lithium? Please log in or register to add a comment. They don�t form solid hydrogencarbonates, but react with nitrogen to form nitrides.
 Source: studylib.net
Source: studylib.net
Oxygen is in group 16/via, which is called the chalcogens, and members of the same group have similar properties. Two electron configurations represent elements that would have similar chemical properties are (2) and (4). Each element within a group has similar physical or chemical properties because of its atom’s outermost electron shell (most chemical properties are dominated by the orbital location of the outermost electron).
 Source: orangesciences.blogspot.com
Source: orangesciences.blogspot.com
Please log in or register to add a comment. Magnesium, calcium, strontium, barium and radium have similar properties to beryllium. So, these elements are placed in 3rd group due to which these elements have similar properties.
 Source: slideplayer.com
Source: slideplayer.com
Magnesium, strontium, and barium belong to group 2a of the periodic table. Magnesium, strontium, and barium belong to group 2a of the periodic table. Its physical and chemical properties are most similar to its heavier homologues strontium and barium.
 Source: numerade.com
Source: numerade.com
The elements calcium, strontium and barium were put in one group and family on the basis of their similar properties. What were those similar properties? The pair of elements that have similar chemical properties are a lithium and magnesium b beryllium and boron c aluminium and magnesium d carbon and nitrogen
 Source: dynamicscience.com.au
Source: dynamicscience.com.au
The initial discovery, which was made by dmitry i. A and b, respectively, are : The elements calcium, strontium and barium were put in one group and family on the basis of their similar properties.
 Source: clutchprep.com
Source: clutchprep.com
Oxygen is in group 16/via, which is called the chalcogens, and members of the same group have similar properties. Why do elements have similar chemical properties? Elements in the group have similar chemical properties.
 Source: youtube.com
Source: youtube.com
The periodic table of elements elements in same column (group) have similar chemical properties. Elements found in group 2 of the periodic table have similar properties to it. Two electron configurations represent elements that would have similar chemical properties are (2) and (4).
 Source: haikudeck.com
Source: haikudeck.com
In general, elements in the same column of the periodic table tend to have similar chemical properties. All of these have the very stable eight valence electron configuration (helium = 2), which is associated with chemical. So, these elements are placed in 3rd group due to which these elements have similar properties.
 Source: brainly.com
Source: brainly.com
Elements in the group have similar chemical properties. When the chemical elements are thus arranged, there is a recurring pattern called the “periodic law” in their properties, in which elements in the same column (group) have similar properties. For example, sodium, potassium, lithium, rubidium, and caesium have similar chemical properties.

Calcium is a chemical element with the symbol ca and atomic number 20. These have two valence electrons in their outermost shell. In general, elements in the same column of the periodic table tend to have similar chemical properties.
 Source: courses.lumenlearning.com
Source: courses.lumenlearning.com
All of these have the very stable eight valence electron configuration (helium = 2), which is associated with chemical. In general, elements in the same column of the periodic table tend to have similar chemical properties. What are three elements that have similar chemical properties to lithium?
What were those similar properties? So, these elements are placed in 3rd group due to which these elements have similar properties. For example, sodium, potassium, lithium, rubidium, and caesium have similar chemical properties.
 Source: slideplayer.com
Source: slideplayer.com
All of these have the very stable eight valence electron configuration (helium = 2), which is associated with chemical. There aren’t any with identical chemical properties (if there were it would be very difficult to isolate them) but the other alkali metals sodium, potassium, rubidium, caesium and francium have similar chemical properties. Each element within a group has similar physical or chemical properties because of its atom’s outermost electron shell (most chemical properties are dominated by the orbital location of the outermost electron).
Also Read :