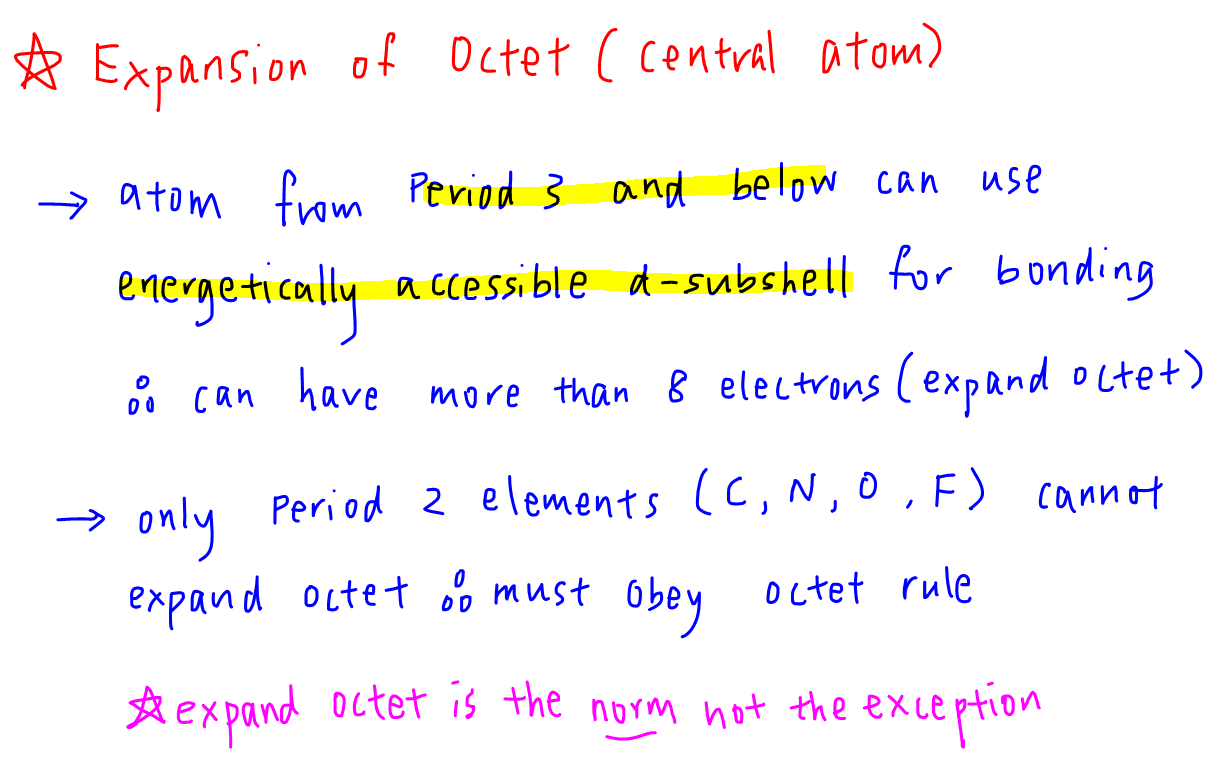
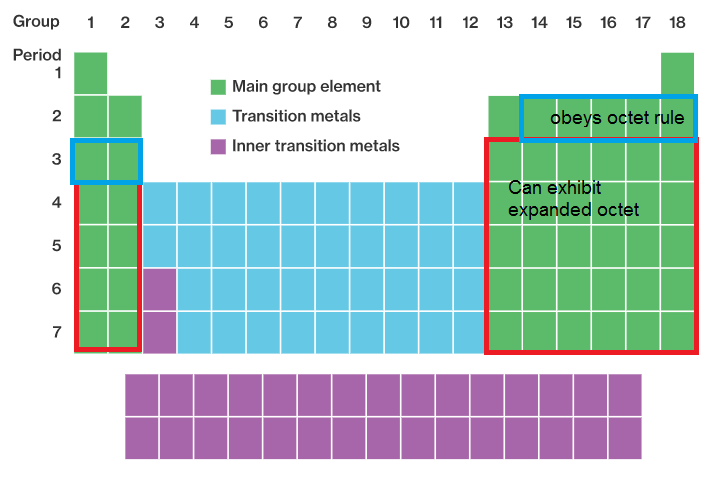
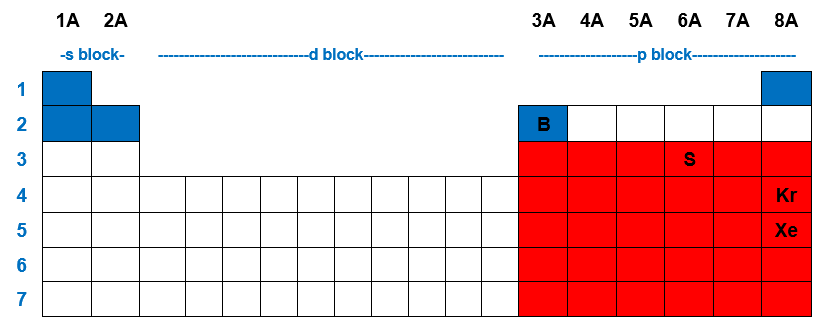
Elements in period 3 and beyond. As per octet rule, atoms bond in a way that they have eight electrons in their valence shell.
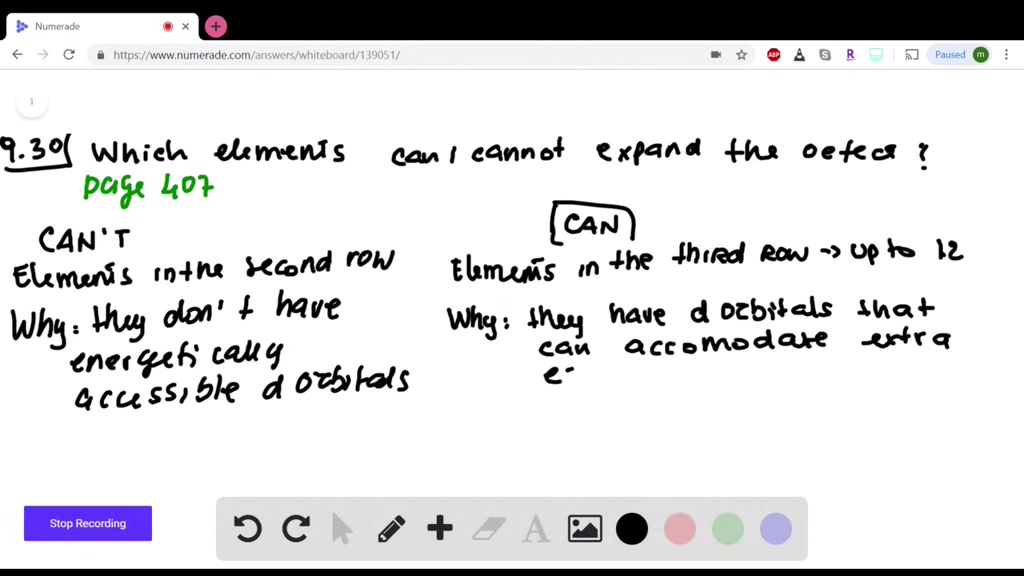
Which Elements Can Have Expanded Octets. I believe br is the only one with an atomic number larger than 10. In the case of octet expansion, the rule is broken. Elements in group 3a and beyond. Elements in group 3a and beyond.
 9.9 Exceptions To The Octet Rule: Odd-Electron Species, Incomplete Octets, & Expanded Octets - Youtube From youtube.com
9.9 Exceptions To The Octet Rule: Odd-Electron Species, Incomplete Octets, & Expanded Octets - Youtube From youtube.com
Related Post 9.9 Exceptions To The Octet Rule: Odd-Electron Species, Incomplete Octets, & Expanded Octets - Youtube :
Main group elements that form more bonds than would be predicted by the octet rule are called hypervalent compounds, and have what is known as an ‘expanded octet,’ meaning that there are more than eight electrons around one atom. Fluorine is more electronegative than sulfur, but hydrogen is not. All the other elements can have ‘depleted octets ‘ or ‘ expanded octets ‘. O only the transition metals.
When an element�s valence shell has enough orbitals to accommodate more than 8 valence electrons, they can form an expanded octet.
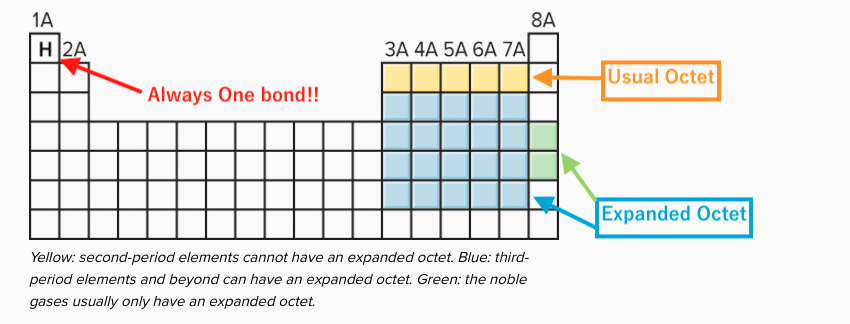
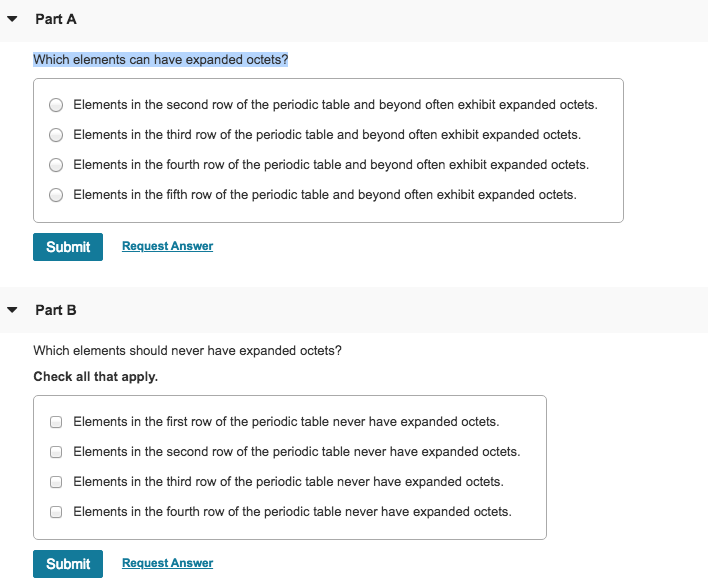
Elements in the first or second row of the periodic table never have expanded octets. Elements in the first row of the periodic table never have expanded octets. N=3 or higher) can form compounds with an expanded octet. Elements in the second row of the periodic table and beyond often exhibit expanded octets. Which elements can have expanded octets? 3) expanded octets, seen in molecules or ions with more than 8 electrons surrounding an atom (asfsub5).
 Source: chemistryguru.com.sg
Source: chemistryguru.com.sg
- odd octets, seen in electron species, molecules, or ions with an odd number of valence electrons (no). This occurs to nonmetals from period 3 to 7. 2) incomplete octets, seen in molecules or ions with fewer than 8 electrons surrounding an atom (bfsub3).
 Source: clutchprep.com
Source: clutchprep.com
Atoms in these periods may follow the octet rule, but there are conditions where they can expand their valence shells to accommodate more than eight electrons. Expanded octet means that an element possesses more than eight electrons in its valance shell. When an element�s valence shell has enough orbitals to accommodate more than 8 valence electrons, they can form an expanded octet.

Electron species, molecules, or ions with off number of electrons (ex. Which elements can have expanded octets? Element with eight number of electrons in its valance shell shows octet.
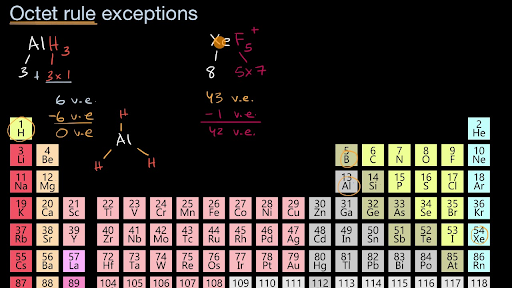
O all of the noble gases. Elements in the third row of the periodic table and beyond often exhibit expanded octets. Atoms in these periods may follow the octet rule, but there are conditions where they can expand their valence shells to accommodate more than eight electrons.
 Source: myweb.liu.edu
Source: myweb.liu.edu
Elements in period 3 and beyond. I brought up p and s but those elements are greater than 10 and since they are in the third period, they contain 3d orbitals and extra electrons can. Molecules or ions with fewer than 8 electrons around an atom (ex.
 Source: thoughtco.com
Source: thoughtco.com
So you find the different categories that can or cannot expand the update. Main group elements that form more bonds than would be predicted by the octet rule are called hypervalent compounds, and have what is known as an ‘expanded octet,’ meaning that there are more than eight electrons around one atom. Elements in the third row of the periodic table never have expanded octets.
 Source: slideplayer.com
Source: slideplayer.com
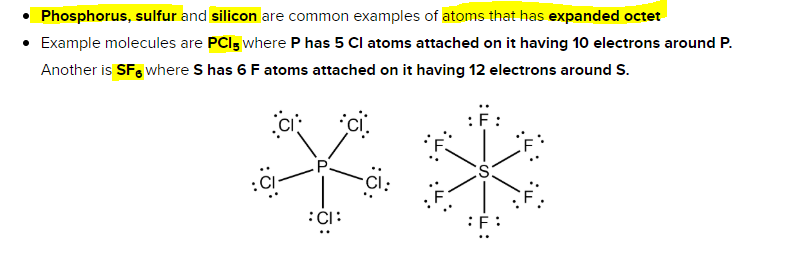
Sulfur, phosphorus, silicon, and chlorine are common examples of elements that form an expanded octet. Periodic table can or cannot expand the oct it. Which elements cannot have expanded octets?
 Source: numerade.com
Source: numerade.com
I believe br is the only one with an atomic number larger than 10. An expanded octet cannot occur with atoms less than atomic number 10 (neon) because there are no d orbitals. Which elements can have expanded octets?
 Source: youtube.com
Source: youtube.com
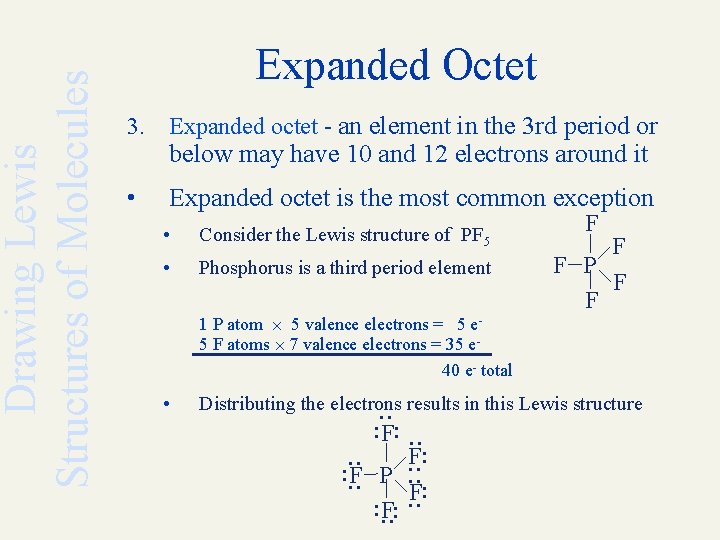
In order to expand octet, the d orbitals must lie low enough to accommodate the extra electrons, and period 3 elements are the only ones capable of doing so. Atoms in these periods may follow the octet rule, but there are conditions where they can expand their valence shells to accommodate more than eight electrons. Phosphorus pentachloride (pcl5) and sulfur hexafluoride (sf6) are examples of molecules that deviate from the octet rule by having more than 8 electrons around the central atom.
 Source: numerade.com
Source: numerade.com
N=3 or higher) can form compounds with an expanded octet. So you find the different categories that can or cannot expand the update. Elements in the third row of the periodic table and beyond often exhibit expanded octets.
 Source: youtube.com
Source: youtube.com
Boron also may form molecules where it only has 6 electrons. • there is an odd number of electrons, like in radicals • there are less than 8 electrons or incomplete octet • there are more than 8 electrons or expanded octet. N=3 or higher) can form compounds with an expanded octet.
 Source: clutchprep.com
Source: clutchprep.com
I brought up p and s but those elements are greater than 10 and since they are in the third period, they contain 3d orbitals and extra electrons can. Boron also may form molecules where it only has 6 electrons. N=3 or higher) can form compounds with an expanded octet.
 Source: chegg.com
Source: chegg.com
Which elements cannot have expanded octets? Which period of elements can have expanded octets? • there is an odd number of electrons, like in radicals • there are less than 8 electrons or incomplete octet • there are more than 8 electrons or expanded octet.
 Source: numerade.com
Source: numerade.com
For example, s f 6 is a stable compound, but s h 6 is not. • there is an odd number of electrons, like in radicals • there are less than 8 electrons or incomplete octet • there are more than 8 electrons or expanded octet. O only the transition metals.
 Source: itprospt.com
Source: itprospt.com
Notice that “expanded octets” only occur in compounds in which the central atom has low electronegativity relative to the ligands. Part a which elements can have expanded octets? Which elements can have expanded octets?
 Source: clutchprep.com
Source: clutchprep.com
O only the transition metals. Elements in period 3 and beyond. Sulfur, phosphorus, silicon, and chlorine are common examples of elements that form an expanded octet.
 Source: studylib.net
Source: studylib.net
An expanded octet cannot occur with atoms less than atomic number 10 (neon) because there are no d orbitals. Periodic table can or cannot expand the oct it. The university of alabama 01:22.
 Source: slidetodoc.com
Source: slidetodoc.com
Elements in the second row of the periodic table and beyond often exhibit expanded octets. 3) expanded octets, seen in molecules or ions with more than 8 electrons surrounding an atom (asfsub5). All the other elements can have ‘depleted octets ‘ or ‘ expanded octets ‘.
 Source: chegg.com
Source: chegg.com
In order to expand octet, the d orbitals must lie low enough to accommodate the extra electrons, and period 3 elements are the only ones capable of doing so. Exceptions to this rule occur when: Third row elements can have an expanded octet.
 Source: clutchprep.com
Source: clutchprep.com
Elements in period 3 and beyond. Notice that “expanded octets” only occur in compounds in which the central atom has low electronegativity relative to the ligands. Exceptions to this rule occur when:
Also Read :





