(1) lithium (2) carbon (3) fluorine (4) neon 8. Elements with complete outermost shell are found in the 8th group on the period table.
Which Element Is Least Likely To Undergo A Chemical Reaction. State the type of reaction listed above. Elements with this configuration have 8 electrons in their outermost shell and 2 for helium. Name the most reactive and the least reactive element. Which element is least likely to undergo a chemical reaction.
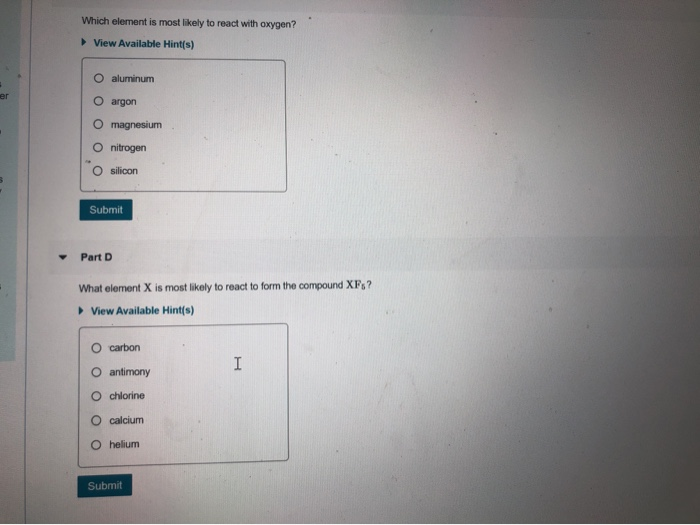 Solved Review Constants1 Periodic Tables Group Oxidaion | Chegg.com From chegg.com
Solved Review Constants1 Periodic Tables Group Oxidaion | Chegg.com From chegg.com
Related Post Solved Review Constants1 Periodic Tables Group Oxidaion | Chegg.com :
The elements toward the bottom left corner of the periodic table are the metals that are the most active in the sense of being the most reactive. Which element is the most likely to react with another element? The two atoms are of the same element. (1) iridium (3) tantalum (2) osmium (4) tungsten 11 which property can be defined as the ability of a substance to be hammered into thin sheets?
The primary difference between metals is the ease with which they undergo chemical reactions.
(1) iridium (3) tantalum (2) osmium (4) tungsten 11 which property can be defined as the ability of a substance to be hammered into thin sheets? Helium is the most perfectly inert of all the elements. Metals at the bottom of a group lose. (1) density (2) electronegativity (3) pressure (4) temperature 9. The two atoms are equally electronegative. It does not undergo chemical reactions of any kind, under any circumstances.
 Source: chemed.chem.purdue.edu
Source: chemed.chem.purdue.edu
A,band care three elements which undergo chemical changes as shown below: Which property is used to determine the degree of polarity between two bonded atoms? What is least likely to form bonds?
 Source: slideplayer.com
Source: slideplayer.com
Metals at the bottom of a group lose. Elements with complete outermost shell are found in the 8th group on the period table. In the 8th group, the elements are generally inert and unreactive.
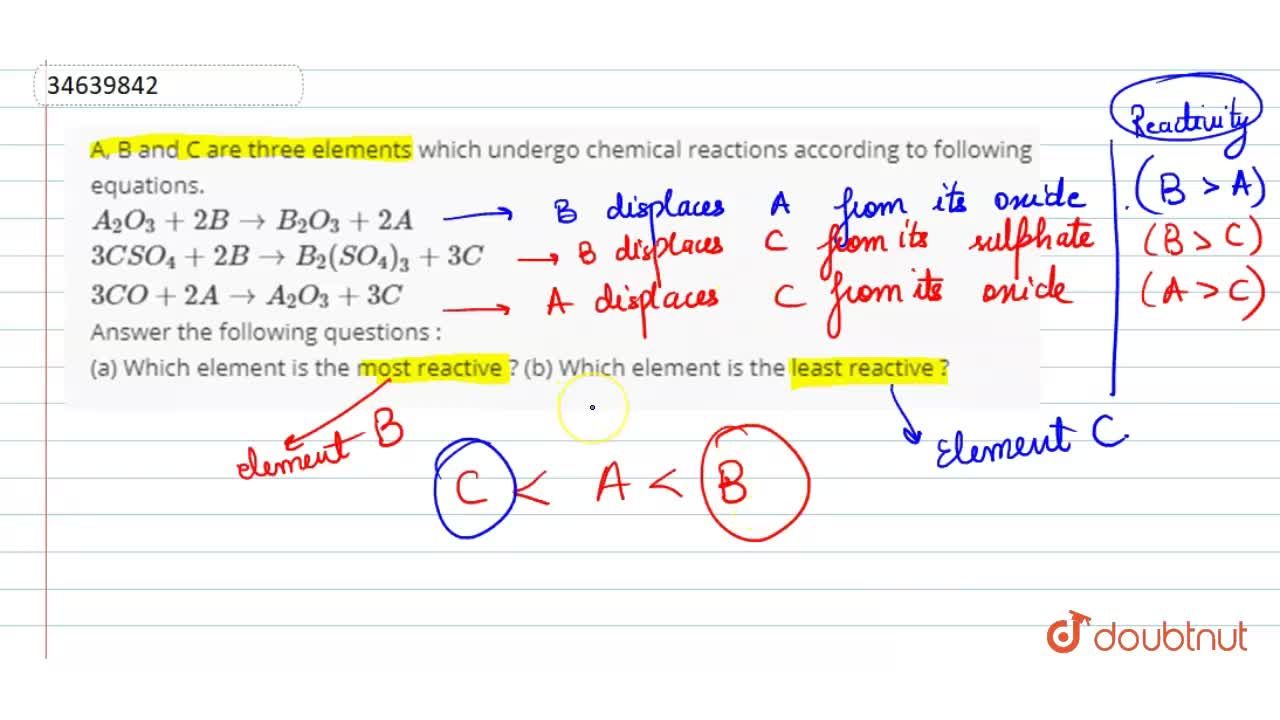 Source: doubtnut.com
Source: doubtnut.com
The bond is part of a tetrahedrally shaped molecule. Which element is least reactive? The two atoms are of the same element.
 Source: britannica.com
Source: britannica.com
Which element is most reactive? The elements toward the bottom left corner of the periodic table are the metals that are the most active in the sense of being the most reactive. Lithium, sodium, and potassium all react.
 Source: studylib.net
Source: studylib.net
9 rows 9 which element is least likely to undergo a chemical reaction? The other elements given in the option do not have 8 electrons in their outermost shell, so they will be quite willing to undergo chemical reactions in order to become stable. (ii) element r is least reactive as it has been replaced.
 Source: toppr.com
Source: toppr.com
Which element is least likely to undergo a chemical reaction? Noble gases are a unique set of elements in the periodic table because they don’t naturally bond with other elements. _____ the diagram below depicts different forms of carbon.
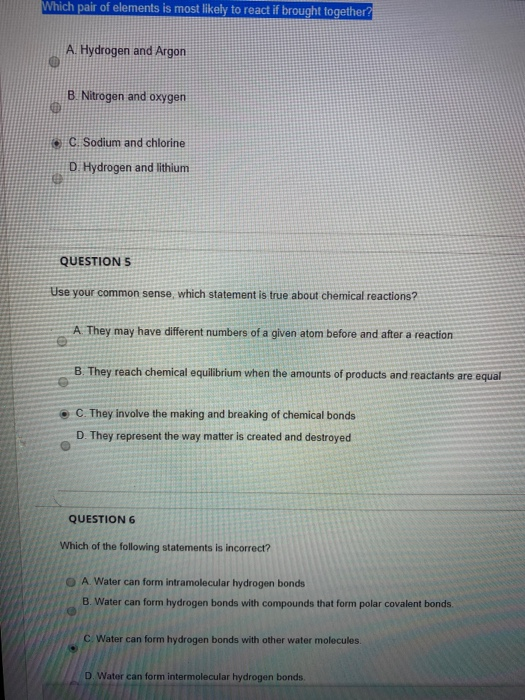 Source: chegg.com
Source: chegg.com
Which element is least likely to undergo a chemical reaction? The primary difference between metals is the ease with which they undergo chemical reactions. Your first response to reduced.
 Source: toppr.com
Source: toppr.com
Which element is least likely to undergo a chemical reaction; (ii) 3cso4 +2b→b2 (so4 )3 +3c. Lithium, sodium, and potassium all react with water, for example.
 Source: brainly.com
Source: brainly.com
Which element is the most likely to react with another element? In the 8th group, the elements are generally inert and unreactive. The two atoms are of the same element.
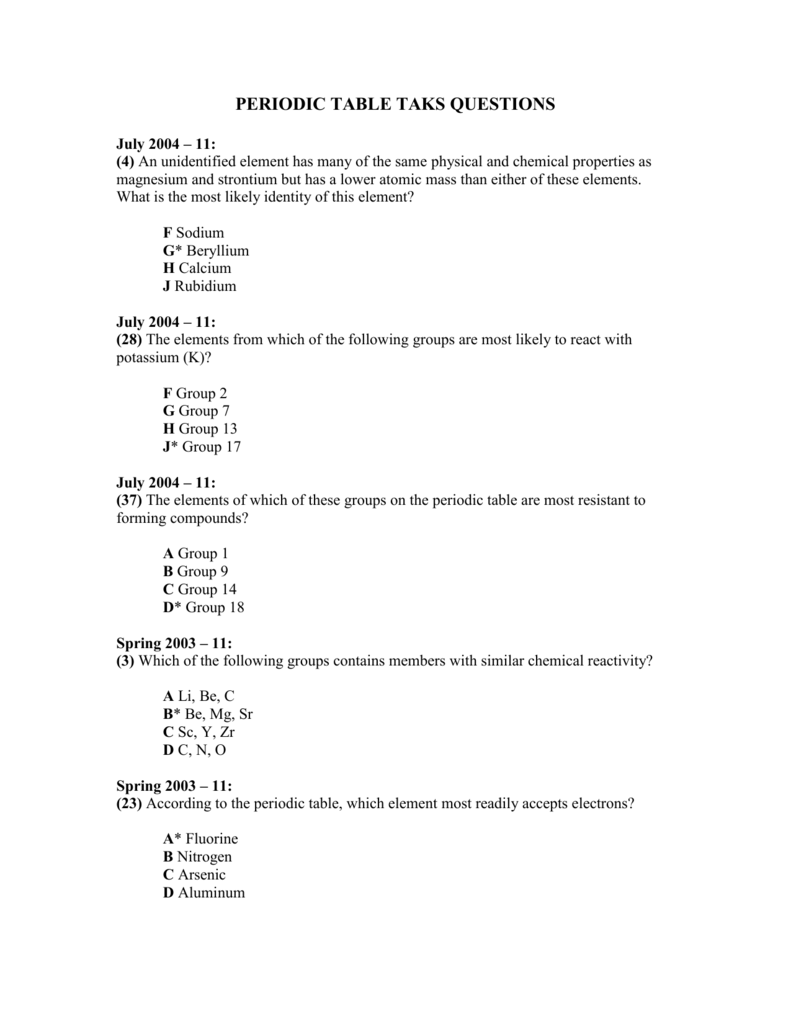 Source: studylib.net
Source: studylib.net
These elements form bonds with one another. The primary difference between metals is the ease with which they undergo chemical reactions. _____ the diagram below depicts different forms of carbon.
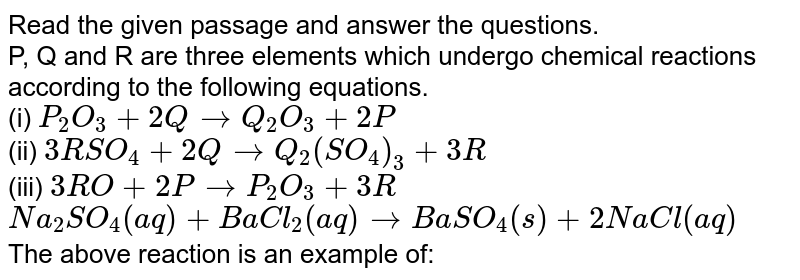 Source: doubtnut.com
Source: doubtnut.com
_____ the diagram below depicts different forms of carbon. The elements toward the bottom left corner of the periodic table are the metals that are the most active in the sense of being the most reactive. A covalent bond between two atoms is likely to be polar if:
 Source: britannica.com
Source: britannica.com
P, q and r are 3 elements which undergo chemical reactions according to the following equations: One of the atoms is much more electronegative than the other. The primary difference between metals is the ease with which they undergo chemical reactions.
 Source: en.wikipedia.org
Source: en.wikipedia.org
In the 8th group, the elements are generally inert and unreactive. State the type of reaction listed above. 15which element is least likely to undergo a chemical reaction?
 Source: chem.libretexts.org
Source: chem.libretexts.org
The primary difference between metals is the ease with which they undergo chemical reactions. The elements toward the bottom left corner of the periodic table are the metals that are the most active in the sense of being the most reactive. P, q and r are 3 elements which undergo chemical reactions according to the following equations:
 Source: britannica.com
Source: britannica.com
Correct answer to the question which of these would not be considered “maintaining homeostasis”? State the type of reaction listed above. Which element is least likely to undergo a chemical reaction?
 Source: quizlet.com
Source: quizlet.com
15which element is least likely to undergo a chemical reaction? _____ the diagram below depicts different forms of carbon. Metals at the bottom of a group lose.
 Source: chegg.com
Source: chegg.com
The primary difference between metals is the ease with which they undergo chemical reactions. A,band care three elements which undergo chemical changes as shown below: 15which element is least likely to undergo a chemical reaction?
 Source: oneclass.com
Source: oneclass.com
(1) lithium (2) carbon (3) fluorine (4) neon Which element is least likely to undergo a chemical reaction? The elements toward the bottom left corner of the periodic table are the metals that are the most active in the sense of being the most reactive.
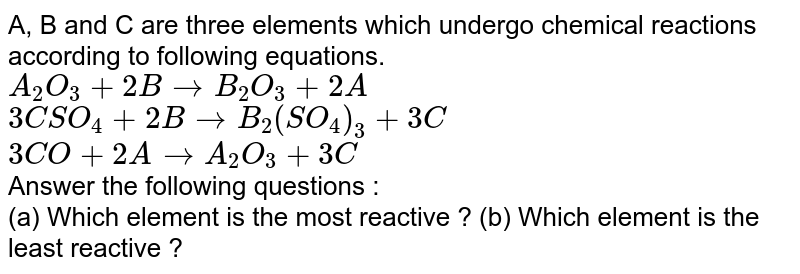 Source: doubtnut.com
Source: doubtnut.com
Circle the element least likely to undergo a chemical reaction. Which element is least likely to undergo a chemical reaction? Name the most reactive and the least reactive element.
 Source: slideplayer.com
Source: slideplayer.com
Which element is least likely to undergo a chemical reaction. Lithium, sodium, and potassium all react. The elements toward the bottom left corner of the periodic table are the metals that are the most active in the sense of being the most reactive.
Also Read :





