Which has lower ionization energy na or k? The third ionization energy is the energy it takes to remove an electron from a 2+ ion.
Which Element Has The Smallest First Ionization Energy. The unity for ionization energy is ev. Since the nuclear charge is necessarily diminished with respect to the valence shell, the alkali metals display the lowest ionization energies, and these energies (reasonably) decrease down the group. Which has lowest first ionization energy? All columns align at the bottom.
 Examples Of Ionization Energy From examples.yourdictionary.com
Examples Of Ionization Energy From examples.yourdictionary.com
Related Post Examples Of Ionization Energy :
Which of the following elements has the greatest first ionization energy? 104 rows the element which has the highest ionization energy is helium with 24.58741 ev. What happens to the values of the successive ionization energies of an element? Because the outer electron in lithium is at a greater distance from the nucleus and experiences a smaller attraction for the nucleus than the electrons in an he atom, it takes less energy to remove this electron from the atom.
The ionization energy decreases from top to bottom in groups, and increases from left to right across a period.
All columns align at the bottom. Potassium (option c) has the smallest first ionization energy from the given elements. The easier the atom can release the electron the lower the energy required to remove it, i.e. Cesiumfrom this trend, cesium is said to have the lowest ionization energy and fluorine is said to have the highest ionization energy (with the exception of helium and neon). Wouldn�t al have the highest third ionisation energy? Which of the following elements has the greatest first ionization energy?
 Source: angelo.edu
Source: angelo.edu
Thus, helium has the largest first ionization energy, while francium has one of the lowest. (2) the 3p electron of aluminium is further from the nucleus compared to the 3s electrons of magnesium. First ionization energy is the energy required to separate one valence electron from an atom in gas phase.
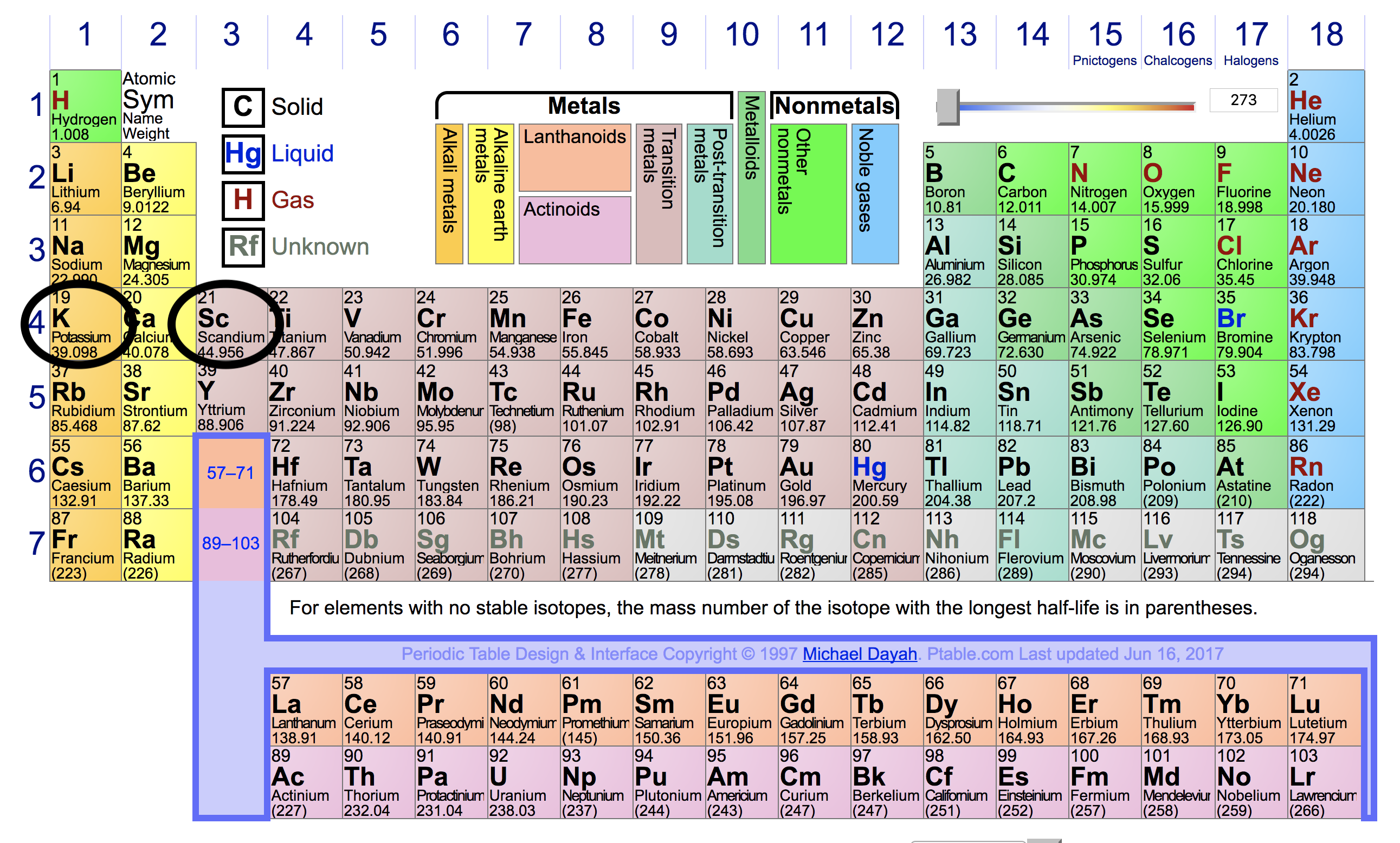 Source: socratic.org
Source: socratic.org
Boron would have the smallest first ionization energy, among boryn, beryllium and chlorine. Which has lowest first ionization energy? Which element has the lowest first ionization energy?

Wouldn�t al have the highest third ionisation energy? The alkali metals have the lowest first ionisation energies in their respective periods of the periodic table because of their low effective nuclear charge and the ability to attain a noble gas configuration by losing just one electron. Furthermore, which group of elements has lowest ionization energy?

The first or initial ionization energy or ei of an atom or molecule is the energy required to remove one mole of electrons from one mole of isolated gaseous atoms or ions. Which element has the smallest ionization energy? Cesiumfrom this trend, cesium is said to have the lowest ionization energy and fluorine is said to have the highest ionization energy (with the exception of helium and neon).

Potassium (option c) has the smallest first ionization energy from the given elements. The first ionization energy of sulfur is less than that of a phosphorus. Which of the following elements has the smallest first ionization energy?
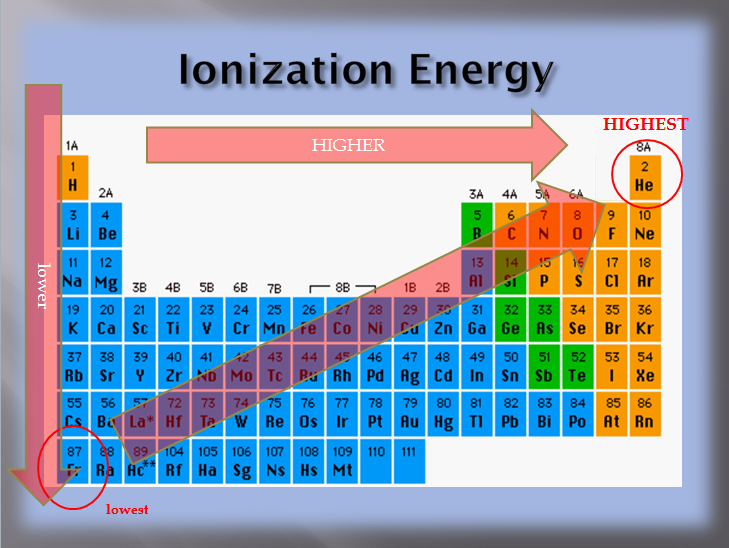 Source: physicalsciencetext.weebly.com
Source: physicalsciencetext.weebly.com
Besides, which element has the smallest first ionization energy? Asked aug 28, 2019 in chemistry by carmenon. Angelo state university › kboudrea.
 Source: clutchprep.com
Source: clutchprep.com
Cesium has atomic number 55 and is in the fifth row of the periodic table. 104 rows the element which has the highest ionization energy is helium with 24.58741 ev. The easier the atom can release the electron the lower the energy required to remove it, i.e.
 Source: quizlet.com
Source: quizlet.com
First ionization energy is the energy required to separate one valence electron from an atom in gas phase. Please note that the elements do not show their natural relation towards each other as in the periodic system. From the first 18 elements only, choose an element which fits the following descriptions:
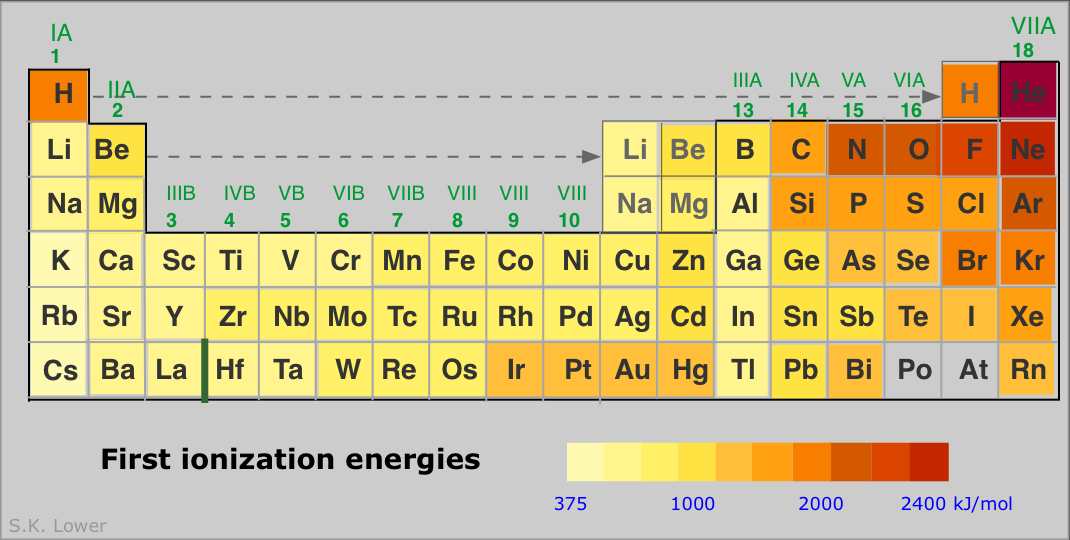 Source: chem.libretexts.org
Source: chem.libretexts.org
(2) the 3p electron of aluminium is further from the nucleus compared to the 3s electrons of magnesium. The ionization energy of k is less than na because although there are more protons, k has 4 electron shells compare to na which has 3. This value uses the pauling scale to measure electronegativity.
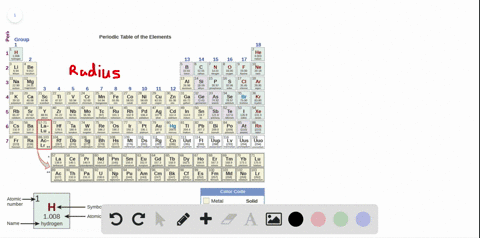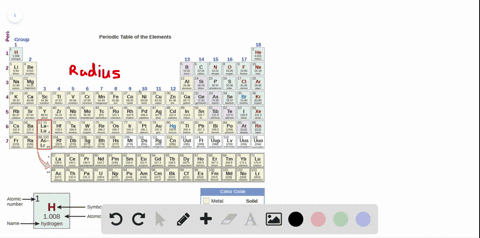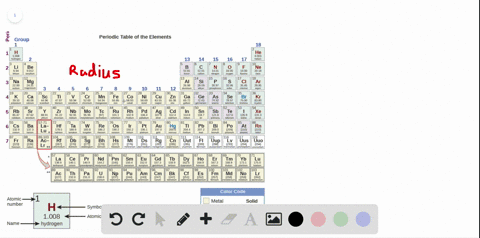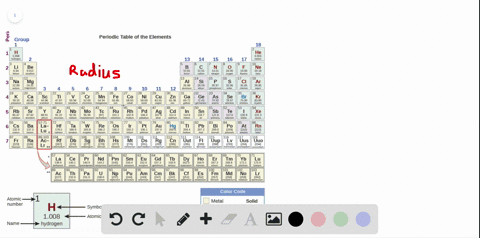 Source: numerade.com
Source: numerade.com
Cesium has atomic number 55 and is in the fifth row of the periodic table. (that means that the atom has already lost two electrons, you are now removing the third.) and 2nd ionization energy is higher than 1st ionization energy, 3rd is higher than 2nd, and so forth. The third ionization energy is the energy it takes to remove an electron from a 2+ ion.

What happens to the values of the successive ionization energies of an element? The first chemical element is cesium and the last one is helium. Which element has the smallest ionization energy?
 Source: examples.yourdictionary.com
Source: examples.yourdictionary.com
Potassium (option c) has the smallest first ionization energy from the given elements. Which element has the lowest ionization energy? Which of the following elements has the greatest first ionization energy?
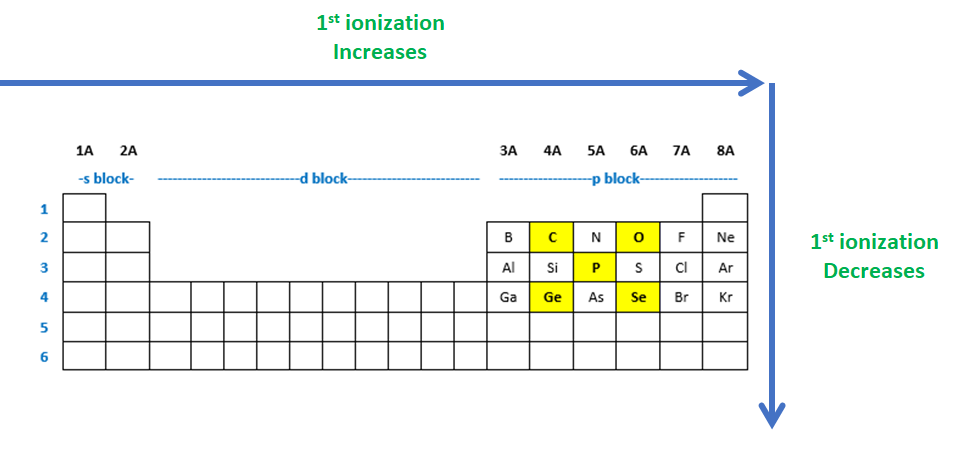 Source: clutchprep.com
Source: clutchprep.com
Which element has the lowest first ionization energy? What happens to the values of the successive ionization energies of an element? Because the outer electron in lithium is at a greater distance from the nucleus and experiences a smaller attraction for the nucleus than the electrons in an he atom, it takes less energy to remove this electron from the atom.
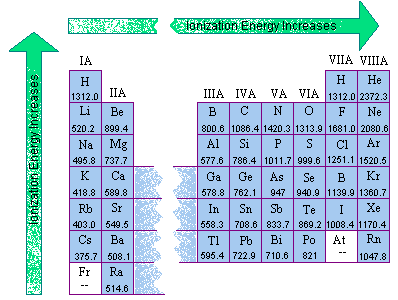 Source: chemed.chem.purdue.edu
Source: chemed.chem.purdue.edu
The first chemical element is cesium and the last one is helium. As indicated in the image attached, the lowest ionization energy�s are those of the alkali earth metals of group ia of the periodic table of the elements. (that means that the atom has already lost two electrons, you are now removing the third.) and 2nd ionization energy is higher than 1st ionization energy, 3rd is higher than 2nd, and so forth.
 Source: angelo.edu
Source: angelo.edu
Which of the following elements has the smallest first ionization energy? The third ionization energy is the energy it takes to remove an electron from a 2+ ion. Which element has the lowest ionization energy?
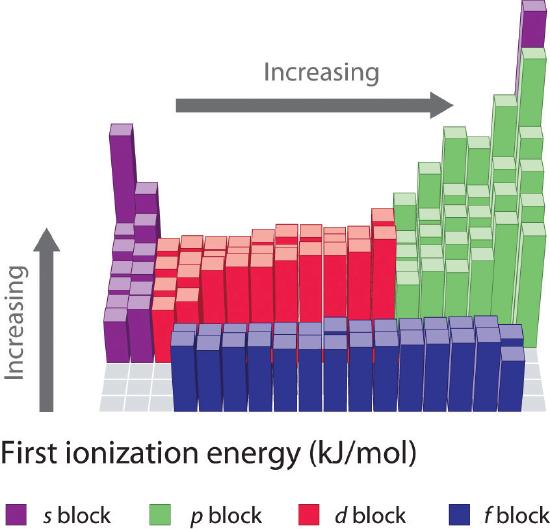 Source: chem.libretexts.org
Source: chem.libretexts.org
What kind of element has low electronegativity? Its radius is large and there is only in one electron in last energy level. Now in the case of na and mg ,na has lower ionization energy and mg has higher.
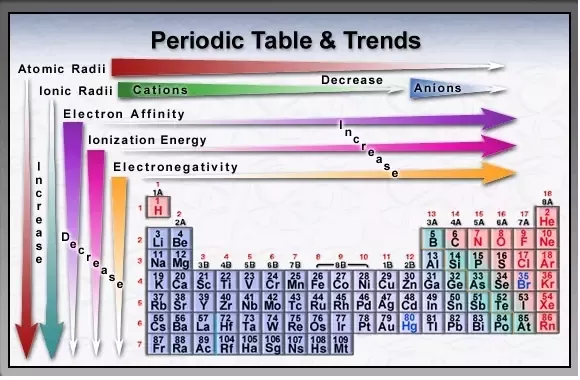 Source: quora.com
Source: quora.com
A reasonable explanation for this fact involves pairing of two electrons in one 3p orbital in sulfur atoms Why does group 1 have the lowest ionization energy? Ionization energy is the energy required to remove an electron from a gaseous atom or ion.
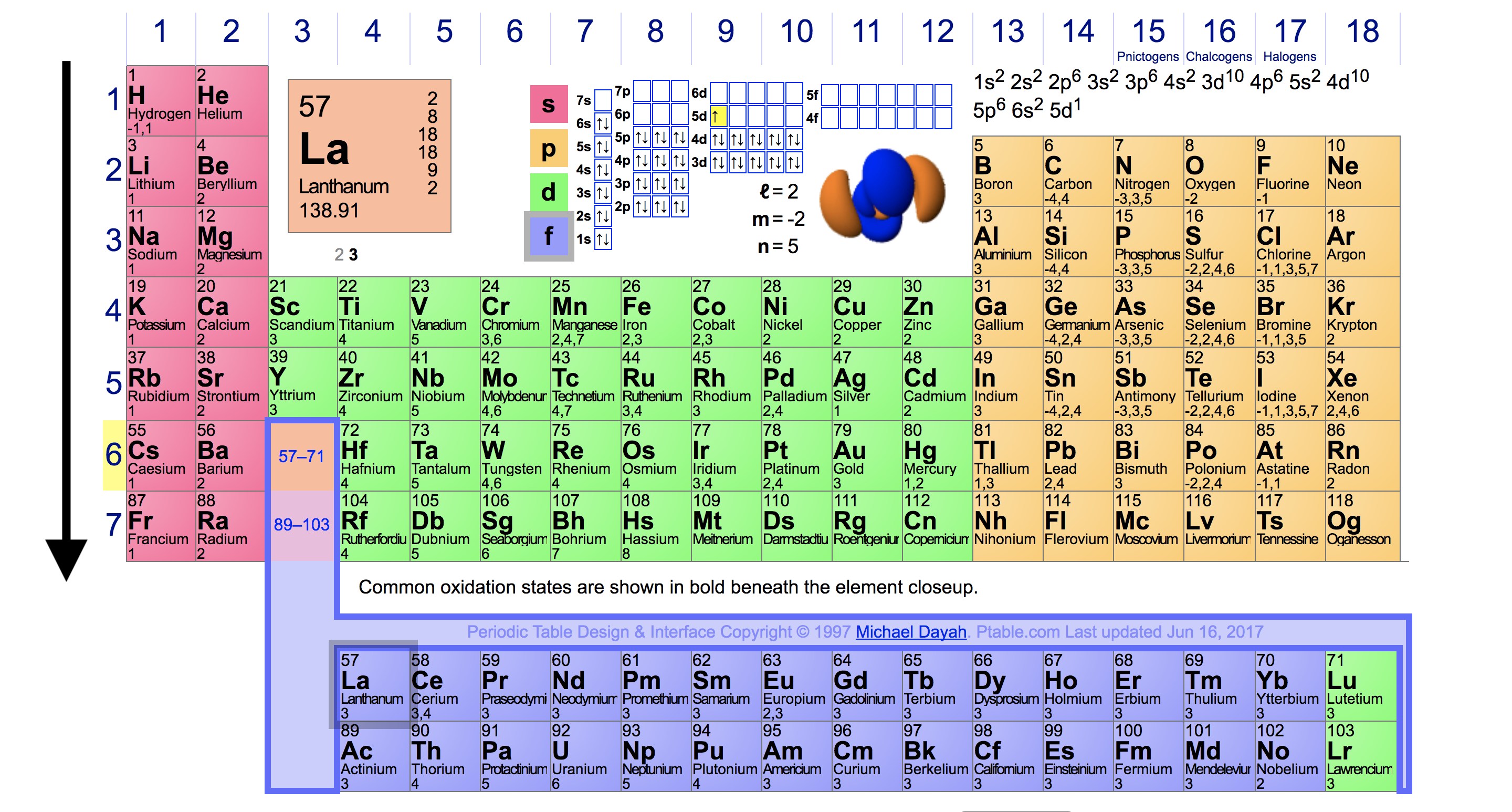 Source: socratic.org
Source: socratic.org
Francium fr (francium) has the lowest ionization energy. The easier the atom can release the electron the lower the energy required to remove it, i.e. Thus, helium has the largest first ionization energy, while francium has one of the lowest.
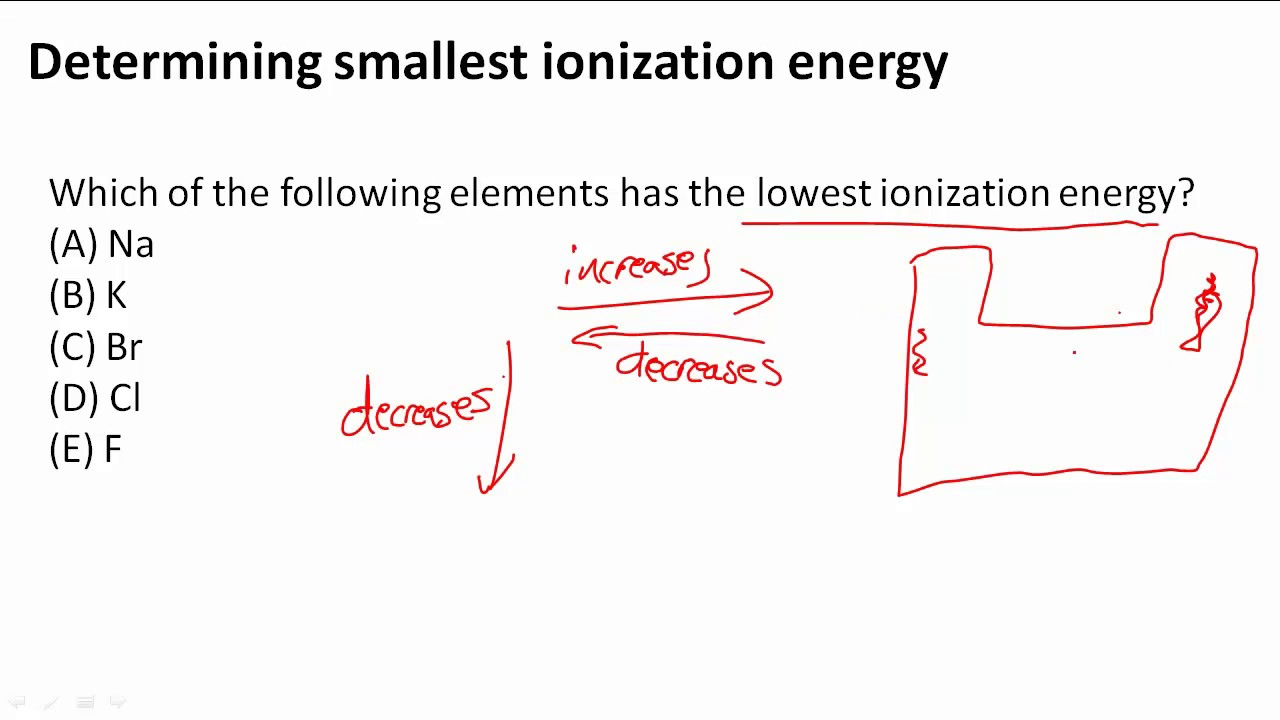 Source: youtube.com
Source: youtube.com
Asked aug 28, 2019 in chemistry by carmenon. The easier the atom can release the electron the lower the energy required to remove it, i.e. The ionization energy decreases from top to bottom in groups, and increases from left to right across a period.
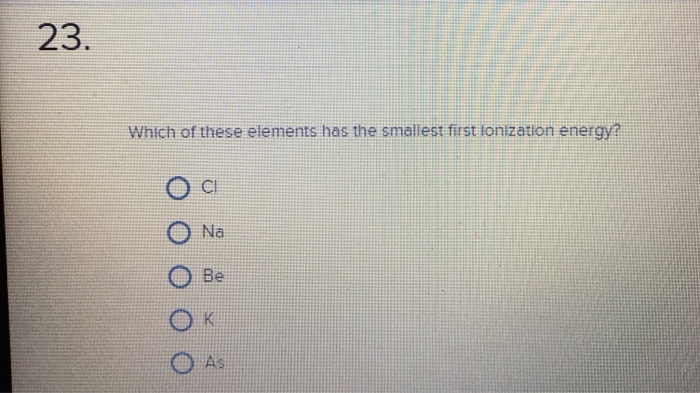 Source: chegg.com
Source: chegg.com
For chemistry students and teachers: Helium thus, helium has the largest first ionization energy, while francium has one of the lowest. Which element has the lowest first ionization energy?
Also Read :





