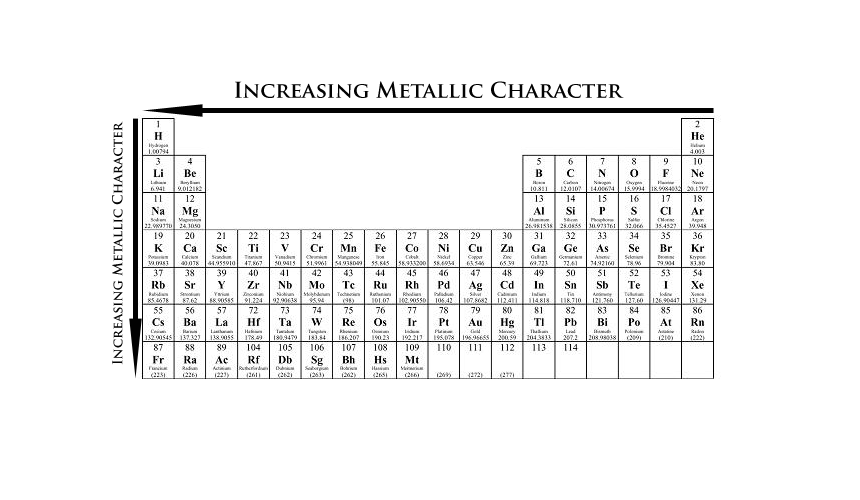
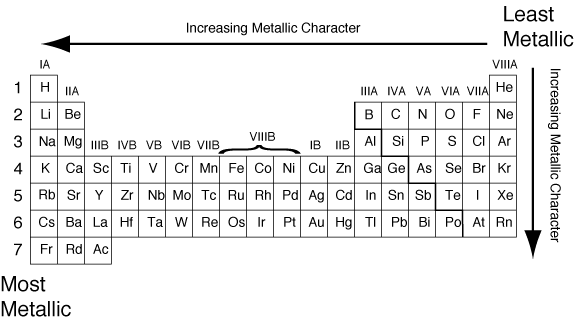
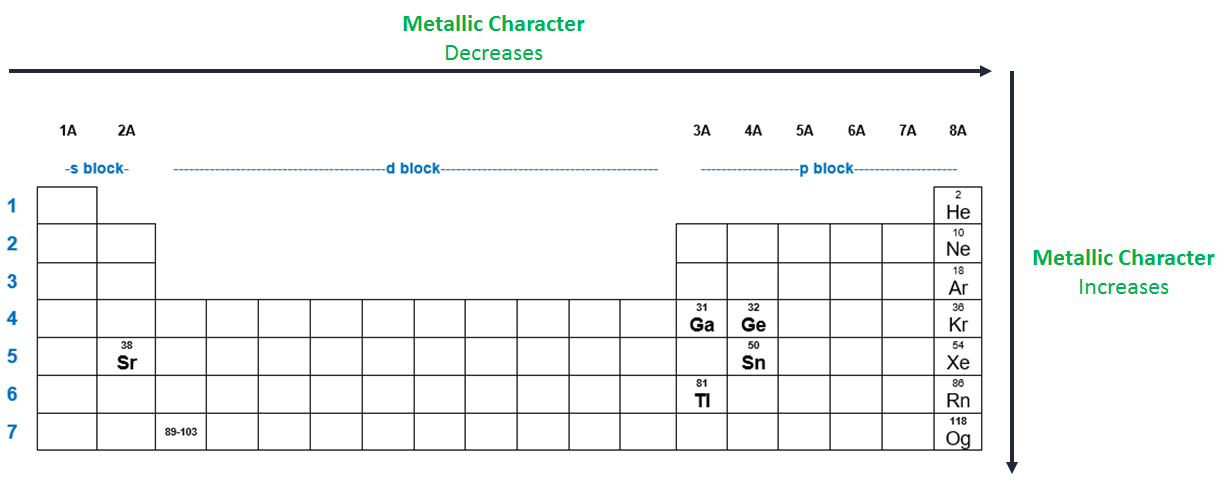
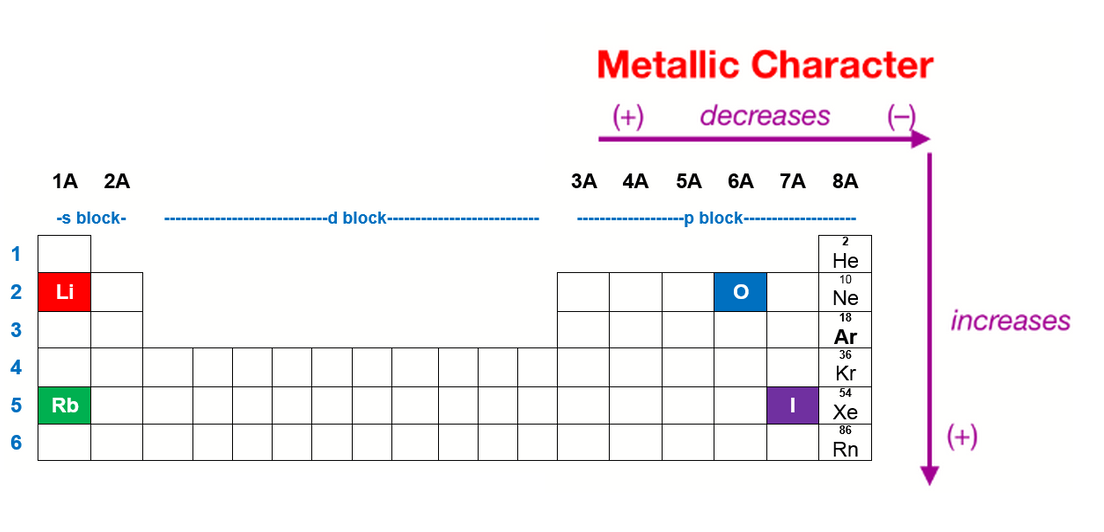
Thus, the elements helium, neon, fluorine,. Now if we analyze the trends in the periodic table metallic character decreases as you move from left to right and from bottom to top of the periodic table.
Which Element Has The Least Metallic Character. Metallic character is the set of properties associated with metals. A) si b) al c) na d) mg Halogens near the top of the periodic table are the least metallic elements, not the noble gases. Arrange the elements silicon, sodium, fluorine, and cesium, in order of decreasing metallic character.
 Please Help Me I Will Give You The Brain Thing And Extra Points. Use The Periodic Table To Predict - Brainly.com From brainly.com
Please Help Me I Will Give You The Brain Thing And Extra Points. Use The Periodic Table To Predict - Brainly.com From brainly.com
Related Post Please Help Me I Will Give You The Brain Thing And Extra Points. Use The Periodic Table To Predict - Brainly.com :
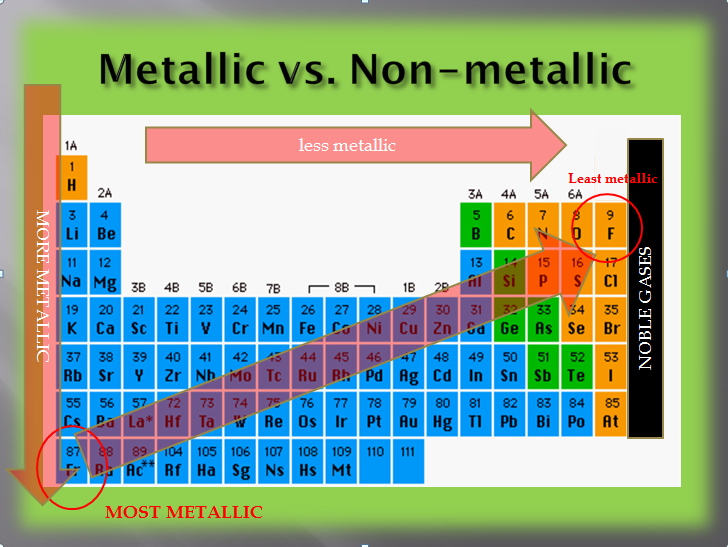
Thus, the elements helium, neon, fluorine,. The elements with the most metallic character are on the left side of the periodic table (except hydrogen). So, fluorine is more electronegative than helium or neon, even though these noble gases are to the right of fluorine on the table. They are located at the right corner uppermost elements.
The electrons of the outermost shell experience less nuclear attraction and so can lose electrons easily thus showing increased metallic character.
Which element has the largest electron affinity? Beside this, what are the characteristics of group 2 elements? The right and uppermost elements on the periodic table. What is the correct name for the compound formed from scandium and silicon? The electrons of the outermost shell experience less nuclear attraction and so can lose electrons easily thus showing increased metallic character. These properties include metallic luster, formation of cations, high electrical and thermal conductivity, and malleability.
 Source: chegg.com
Source: chegg.com
So, fluorine is more electronegative than helium or neon, even though these noble gases are to the right of fluorine on the table. The right and uppermost elements on the periodic table. Halogens near the top of the periodic table are the least metallic elements, not the noble gases.
 Source: sciencenotes.org
Source: sciencenotes.org
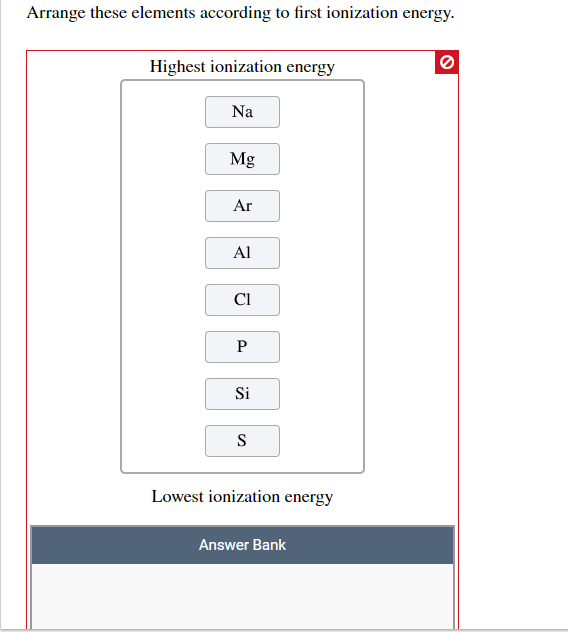
(i.e., from most metallic to least) a) cesium > sodium > silicon > fluorine b) fluorine > silicon > sodium > cesium c) sodium > cesium > silicon > fluorine d) fluorine >. Chlorine is right below fluorine, making it the element with the fifth highest metallic character. As you move down the column, the melting and boiling points _____ and the density _____.
 Source: 170188733453308075.weebly.com
Source: 170188733453308075.weebly.com
As an example, the metallic character of beryillium (4), would not be as great as the metallic character of barium (56). Silicon carbide, sic, is prepared by heating silicon dioxide in the presence of graphite. Arrange the elements silicon, sodium, fluorine, and cesium, in order of decreasing metallic character.
 Source: physicalsciencetext.weebly.com
Source: physicalsciencetext.weebly.com
As you move down the column, the melting and boiling points _____ and the density _____. Thus b e has least metallic character. A metallic compound would have.
 Source: theptproject.weebly.com
Source: theptproject.weebly.com
If we look at the periodic table group 17 and group 18 have the least or lowest metallic character. Certainly, na because it is in the 1st group of the periodic table. Silicon carbide, sic, is prepared by heating silicon dioxide in the presence of graphite.
 Source: youtube.com
Source: youtube.com
It has the lowest electronegativity which means it easier loses electrons. Use the periodic table to predict which element has the least metallic character. A) si b) al c) na d) mg
 Source: slidetodoc.com
Source: slidetodoc.com
Which group has least metallic character? Use the periodic table to predict which element has the least metallic character. Halogens near the top of the periodic table are the least metallic elements, not the noble gases.

This is because the loss of electrons becomes easier as the atomic radius increases and the attraction between the nucleus and valence electrons becomes weaker as the distance between them increases. Which element has the largest electron affinity? A) si b) al c) na d) mg
 Source: courses.lumenlearning.com
Source: courses.lumenlearning.com
Metallic character is mostly dependent on an. They are located at the right corner uppermost elements. Thus, the elements helium, neon, fluorine,.
 Source: clutchprep.com
Source: clutchprep.com
They are located at the right corner uppermost elements. Which group has least metallic character. Metallic character is mostly dependent on an element’s ability to do away its valence electrons.
 Source: chegg.com
Source: chegg.com
Which element has the least metallic character. Li has the least metallic character of the five elements you listed. Use the periodic table to predict which element has the least metallic character.
 Source: brainly.com
Source: brainly.com
If we look at the periodic table group 17 and group 18 have the least or lowest metallic character. Which of the following period 3 elements has the least metallic character? Name an element that has 5.
 Source: clutchprep.com
Source: clutchprep.com
Halogens near the top of the periodic table are the least metallic elements, not the noble gases. Which is the correct formula for the compound formed from magnesium and sulfur? Metallic character is a periodic table trend.
 Source: slideplayer.com
Source: slideplayer.com
They are located at the right corner uppermost elements. Now if we analyze the trends in the periodic table metallic character decreases as you move from left to right and from bottom to top of the periodic table. Halogens near the top of the periodic table are the least metallic elements, not the noble gases.
 Source: study.com
Source: study.com
Certainly, na because it is in the 1st group of the periodic table. A) si b) al c) na d) mg If we look at the periodic table group 17 and group 18 have the least or lowest metallic character.
 Source: xaktly.com
Source: xaktly.com
It has the lowest electronegativity which means it easier loses electrons. If we look at the periodic table group 17 and group 18 have the least or lowest metallic character. Which group has least metallic character.
 Source: clutchprep.com
Source: clutchprep.com
Beside this, what are the characteristics of group 2 elements? Which element has the least metallic characteristics? Metallic character is a periodic table trend.
 Source: chemistrybytes.com
Source: chemistrybytes.com
Which element has the least metallic characteristics? For eg helium , neon ,. Which of the following period 3 elements has the least metallic character?
 Source: thefactfactor.com
Source: thefactfactor.com
Cesium metallic character increases form right to left across a period on the periodic table, and from top to bottom down a group. Metallic character is the set of properties associated with metals. It has the lowest electronegativity which means it easier loses electrons.
 Source: slideplayer.com
Source: slideplayer.com
- which element has the least metallic character?* a) sodium (na) b) beryllium (be) c) potassium (k) d) magnesium (mg) If we look at the periodic table group 17 and group 18 have the least or lowest metallic character. What is the correct name for the compound formed from scandium and silicon?
Also Read :





