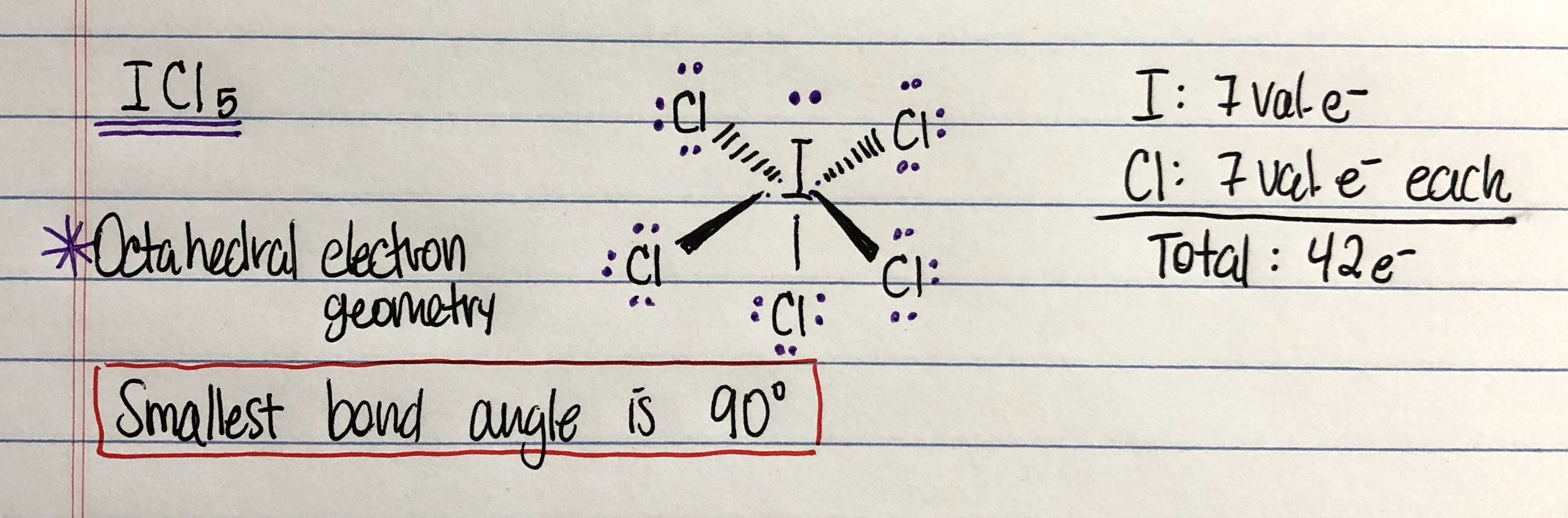The halogen elements have seven valence electrons in their outermost electron shell. Number of valence electrons = main group number (neutral atoms) the main group number of an element can be found in its periodic table column.
Which Element Has 7 Valence Electrons. Halogens have 7 valence electrons. Calcium is a group 2 element with two valence electrons. The valence electrons of an element can be found out by closely examining the vertical column in which the elements are grouped in. Alkali metals have one valence electron.
 Valence Electrons And Energy Levels Of Atoms Of Elements - Video & Lesson Transcript | Study.com From study.com
Valence Electrons And Energy Levels Of Atoms Of Elements - Video & Lesson Transcript | Study.com From study.com
Related Post Valence Electrons And Energy Levels Of Atoms Of Elements - Video & Lesson Transcript | Study.com :
Which group contains elements with two valence electrons? Calcium is a group 2 element with two valence electrons. Alkali metals have eight valence electrons. The valence electron has to be determined by following a few steps.
119 rows elements valence electrons;
Fluorine has atomic number 9. Nitrogen has five valence electrons. The electron configuration is one of them. Fluorine has 7 valence electrons. Therefore, when these elements can receive an electron from another atom, they form very stable compounds since their outermost shell is full. Number of valence electrons = main group number (neutral atoms) the main group number of an element can be found in its periodic table column.
 Source: thinglink.com
Source: thinglink.com
Also, shells don�t stack neatly one on top of another, so don�t always assume an element�s valence is determined by the number of electrons in its outer shell. Which element has 2 energy levels and 7 valence electrons? 112 rows carbon has 6 protons, 6 neutrons and 6 electrons:
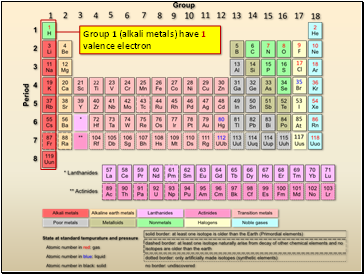 Source: sliderbase.com
Source: sliderbase.com
The halogen elements have seven valence electrons in their outermost electron shell. 112 rows carbon has 6 protons, 6 neutrons and 6 electrons: We have shown the valence electrons of the elements for which reliable data is available.
 Source: studylib.net
Source: studylib.net
Here is a table of element valences. The element chlorine has 7 valence electrons, but a chloride ion gains an electron. When electrons are found in lower energy levels and are in a stable orientation we say that these electrons are in.
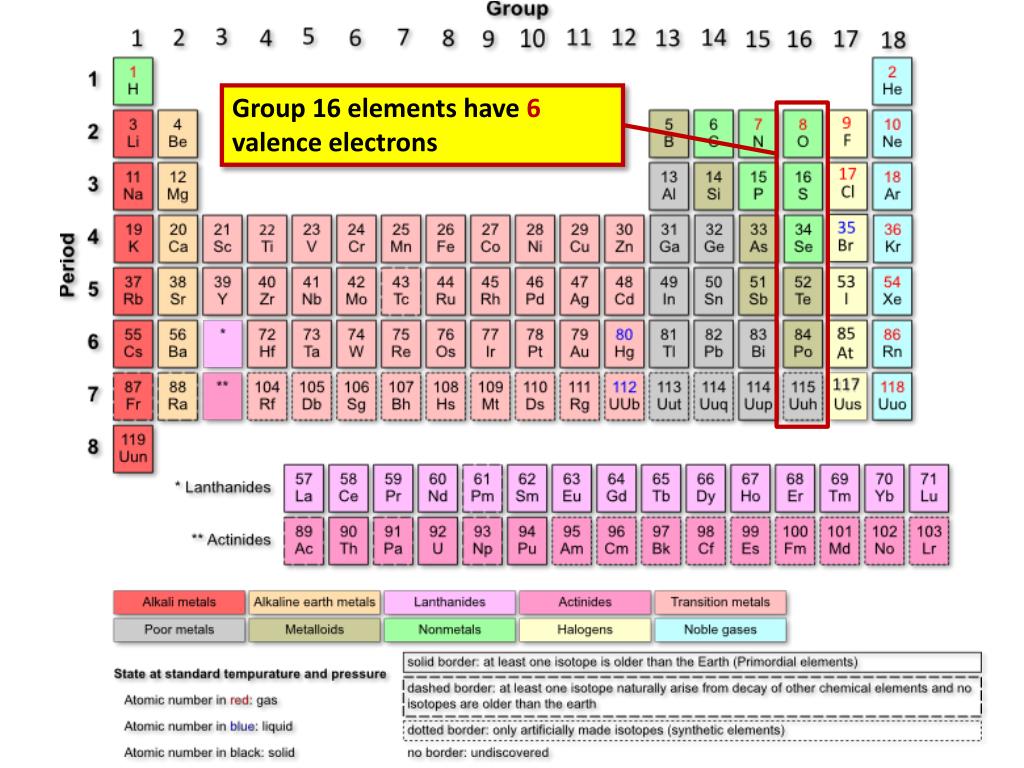 Source: slideserve.com
Source: slideserve.com
It is not possible to determine the valence electron without electron configuration. The electron configuration is one of them. We have shown the valence electrons of the elements for which reliable data is available.
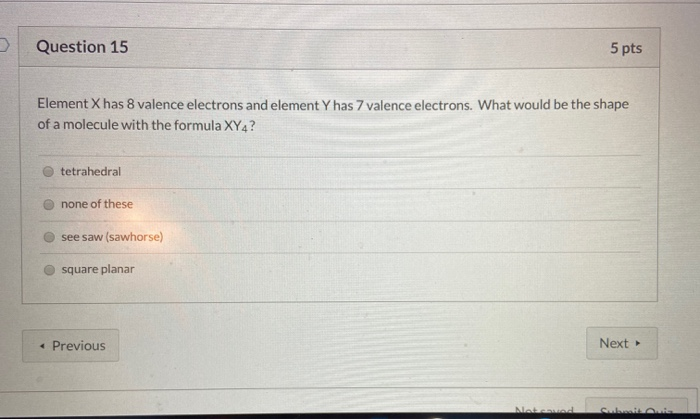 Source: chegg.com
Source: chegg.com
Alkali metals have seven valence electrons. Carbon, for instance, belongs to group 4 and has four valence electrons. (it is number 7 o.
 Source: wikihow.com
Source: wikihow.com
B) 3s13p6 c) 3s13p43d2 d) 3s23p43d1 an atom has the electron configuration 1s22s22p63s23p5. The two electrons in the 4s orbital are obvious valence electrons, so a valence state of 2+ is possible. Does period 4 have 7 valence electrons?
 Source: chegg.com
Source: chegg.com
119 rows elements valence electrons; Alkali metals have eight valence electrons. What electron configuration could represent the outermost principal energy level of this atom in the ground state?
 Source: studylib.net
Source: studylib.net
The electronic configuration of nitrogen is (2,,8,,7.) chlorine has (7) electrons present in its valence shell. Carbon, for instance, belongs to group 4 and has four valence electrons. Valence electrons are those electrons which are present in the outermost shell of an atom.
 Source: wikihow.com
Source: wikihow.com
Elements in the second row (lithium through neon) will have valence electrons in the second energy level with a principal quantum number of 2. (it is number 7 o. A nitrogen (n) b neon (ne) c lithium (li) d chlorine (ci) 1 see answer advertisement
 Source: mcqpoint.com
Source: mcqpoint.com
Nitrogen has five valence electrons. The outer most electrons are called. Alkali metals have two valence electrons.
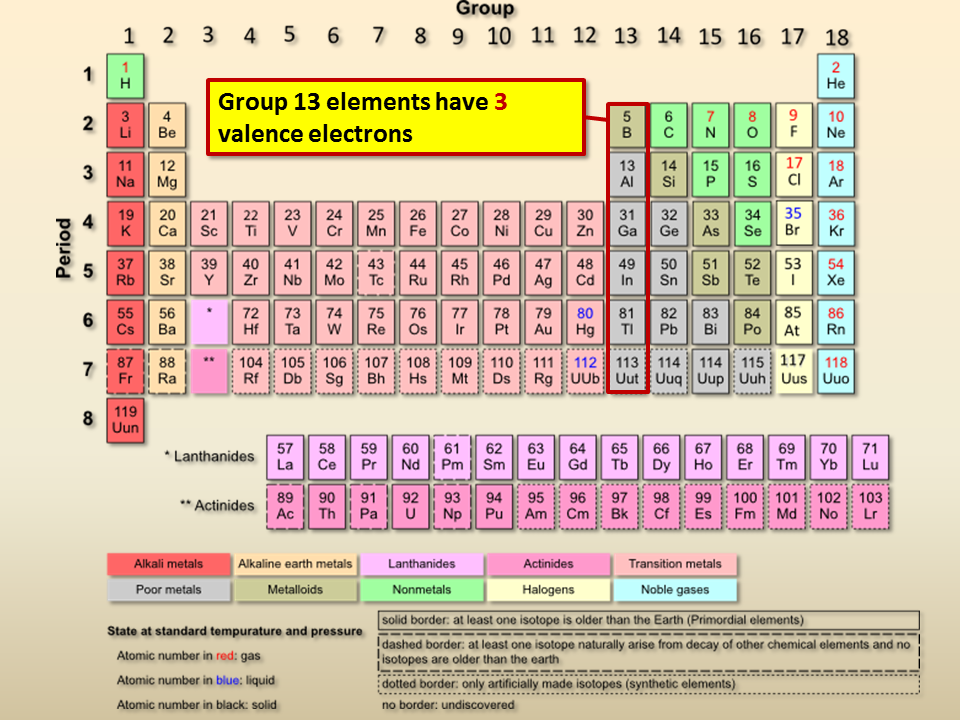
The elements in this family are fluorine, chlorine, bromine, iodine, and astatine. By looking at the group number that is given we can identify the number of valence electrons that an element which is listed under that specific column has. They are never found free in nature.

Any element in the halogen group will have seven valence electrons. 112 rows carbon has 6 protons, 6 neutrons and 6 electrons: Alkali metals have eight valence electrons.
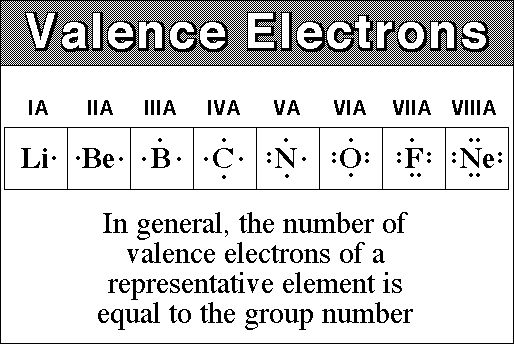 Source: socratic.org
Source: socratic.org
119 rows elements valence electrons; Look at a periodic table of the elements, and you’ll find that nitrogen (and only nitrogen) has precisely seven protons. Its electron configuration is [ar] 3d5 4s2.
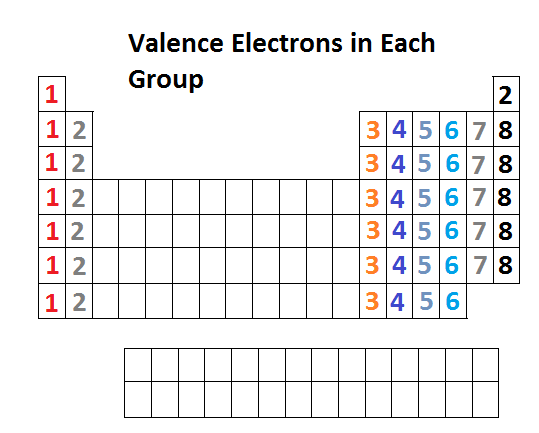 Source: socratic.org
Source: socratic.org
The electron configuration is one of them. Oxygen belongs to group 6. Manganese is a transition metal, meaning that it can have more than one valence state.
 Source: youtube.com
Source: youtube.com
The group 1 7 atoms have 7 valence electrons the group 1 8 atoms have 8 valence electrons with the exception of helium which has 2 electrons the transition metals are more difficult to determine the number of valence electrons. They are never found free in nature. Halogens have 7 valence electrons.
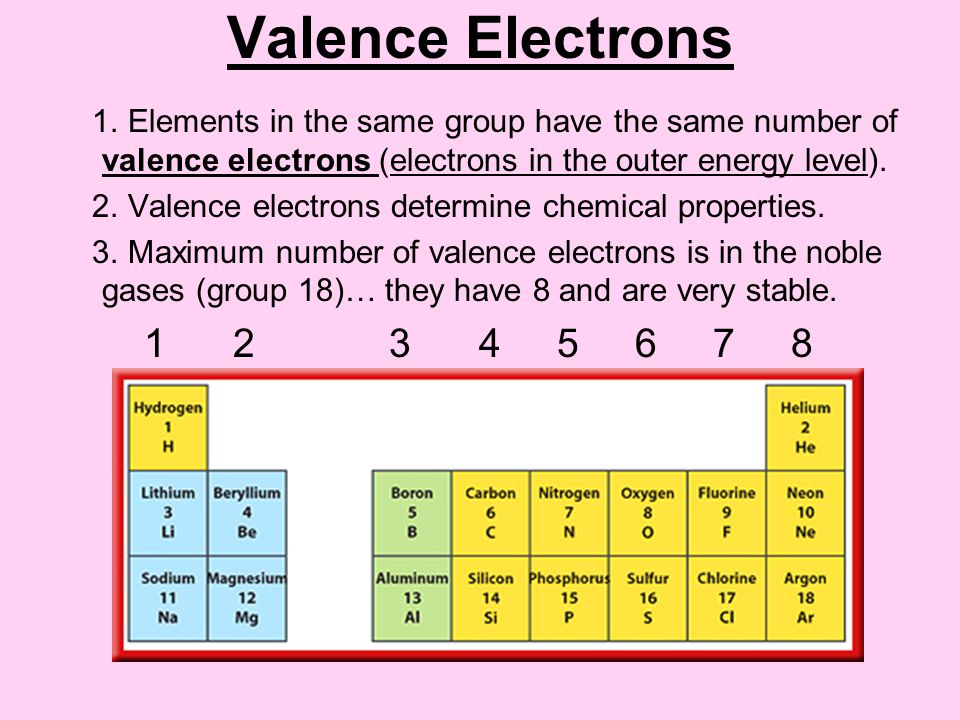 Source: slideplayer.com
Source: slideplayer.com
Which element has 7 valence electrons 1 see answer advertisement advertisement sheilyvalencia203 is waiting for your help. Oxygen belongs to group 6. Fluorine has 7 valence electrons.
 Source: mystorybook.com
Source: mystorybook.com
119 rows elements valence electrons; It has (17) electrons distributed in (3) energy shells. These elements include fluorine, chlorine, bromine, iodine, and astatine.
 Source: study.com
Source: study.com
Which element has 2 energy levels and 7 valence electrons? Which group contains elements with two valence electrons? Therefore, when these elements can receive an electron from another atom, they form very stable compounds since their outermost shell is full.
 Source: chemistry.stackexchange.com
Source: chemistry.stackexchange.com
Knowing the electron configuration in the right way, it is very easy to determine the valence electrons of all the elements. The elements in this family are fluorine, chlorine, bromine, iodine, and astatine. Fluorine has atomic number 9.
 Source: chem.uiuc.edu
Source: chem.uiuc.edu
Oxygen belongs to group 6. When electrons are found in higher energy. Its electron configuration is [ar] 3d5 4s2.
Also Read :