Total valence electrons ‘=’ 5 + 4(6) ‘=’ 29. Hence it can have more than 8 electrons involved in its bonding.
Which Element Has 5 Valence Electrons. What family has 5 valence electrons? Thus phosphorus has 5 valence valence electrons. Therefore, elements whose atoms can have the same number of valence electrons are grouped together in the periodic table of the elements. The valence electron has to be determined by following a few steps.
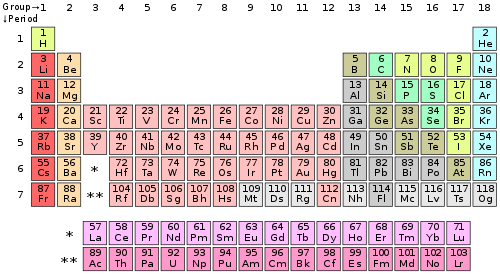 1.3: Valence Electrons And Open Valences - Chemistry Libretexts From chem.libretexts.org
1.3: Valence Electrons And Open Valences - Chemistry Libretexts From chem.libretexts.org
Related Post 1.3: Valence Electrons And Open Valences - Chemistry Libretexts :
The electronic configuration of phosphorous is 2,8,5. Elements having seven valence electrons are present in group (17) of the periodic table. Which one of the elements below has 5 valence electrons in its lewis symbol? The third shell is the outer valence shell, so it has 5 valence electrons.
Elements having seven valence electrons are present in group (17) of the periodic table.
All group 15 elements have 5 valence electrons. The elements of the group 15 (column) va of the periodic table all have electron configurations of s2p3, giving them five valence electrons. This is because such an atom has only a single valence. These elements easily accept an electron to complete its valence shell. These elements include nitrogen (n), phosphorus (p), arsenic (as), antimony (sb) and bismuth (bi). Elements having seven valence electrons are present in group (17) of the periodic table.
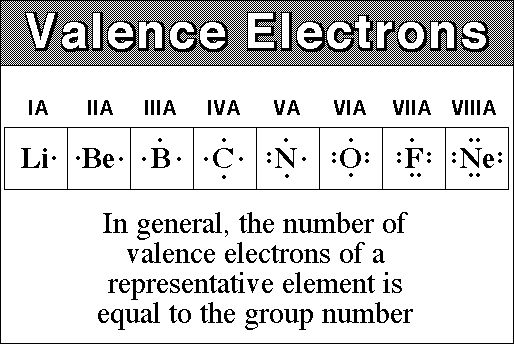 Source: socratic.org
Source: socratic.org
What family has 5 valence electrons? The valence electron has to be determined by following a few steps. Which one of the following bonds is the most polar one of the set?

Source: slideplayer.com
These elements include nitrogen (n), phosphorus (p), arsenic (as), antimony (sb), and bismuth (bi). A neutral phosphorus atom has 15 total electrons. They tend to share electrons when they bond.
 Source: clutchprep.com
Source: clutchprep.com
Knowing the electron configuration in the right way, it is very easy to determine the valence electrons of all the elements. By “five orbital shells” i assume you mean that at least some of the valence electrons of the element are in the 5th energy level. Examples include hydrogen (h), lithium (li), and sodium (na).
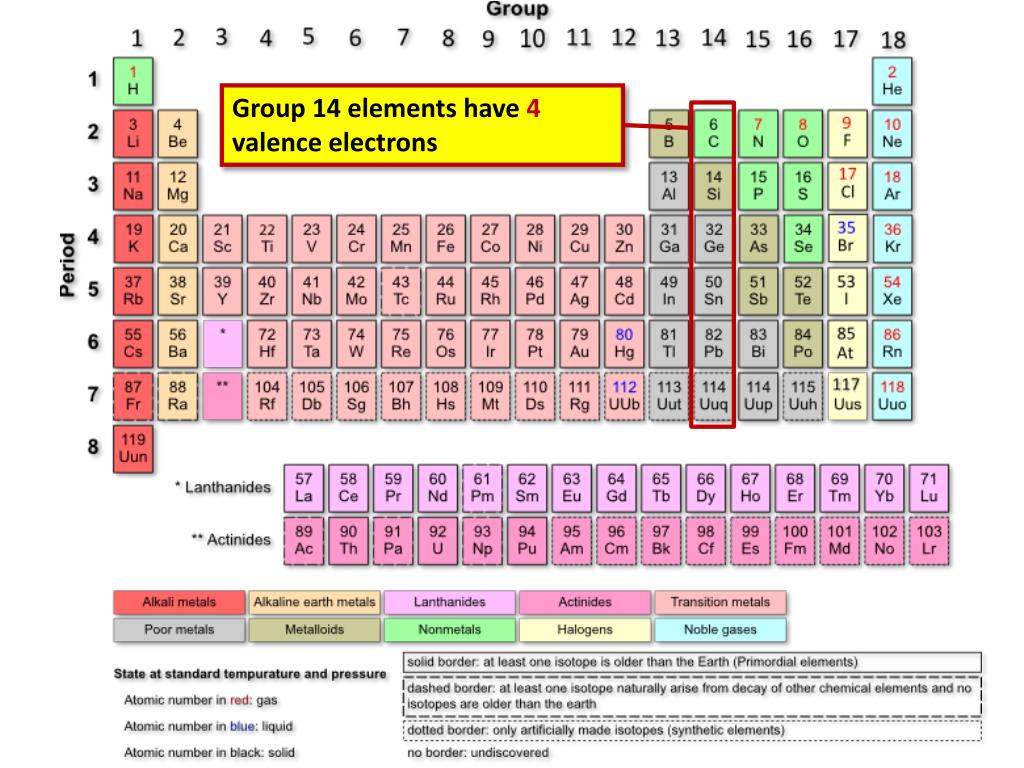 Source: slideserve.com
Source: slideserve.com
Two electrons can go into first shell, eight in the second shell, and it has five more in the third shell. Hence it can have more than 8 electrons involved in its bonding. So go to that group in the periodic table and go to the last element in the group and that has the highest atomic # the nitrogen family can form five covalent bonds,
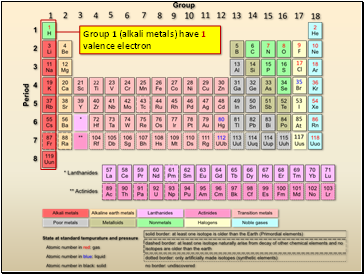 Source: sliderbase.com
Source: sliderbase.com
Which one of the elements below has 5 valence electrons in its lewis symbol? The valence electron has to be determined by following a few steps. All group 15 elements have 5 valence electrons.
 Source: study.com
Source: study.com
Which group is it in? Thus it has three shells and 5 electrons in its outermost shell. An element has 5 valency electrons.
 Source: study.com
Source: study.com
So, iodine can use all of these electrons in chemical bonding: Elements having seven valence electrons are present in group (17) of the periodic table. The electron is one of the most important factors in determining how an atom will react with another atom or.
 Source: wikihow.com
Source: wikihow.com
This group of elements consists of halogens. Which group is it in? So go to that group in the periodic table and go to the last element in the group and that has the highest atomic # the nitrogen family can form five covalent bonds,

By “five orbital shells” i assume you mean that at least some of the valence electrons of the element are in the 5th energy level. Valence electrons can greatly impact the properties of atoms of the same element. Arsenic is in group 5 and period 4.
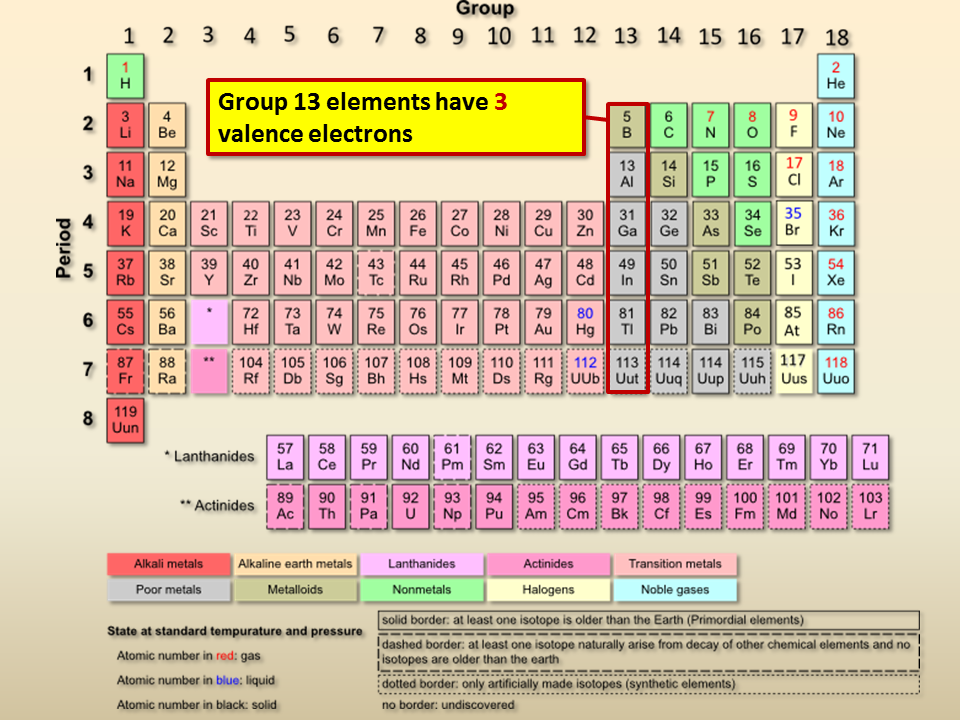
Elements having seven valence electrons are present in group (17) of the periodic table. The formal charge on the oxygen atom in the hydronium ion is a. These elements easily accept an electron to complete its valence shell.
 Source: chemistry.stackexchange.com
Source: chemistry.stackexchange.com
The third shell is the outer valence shell, so it has 5 valence electrons. So go to that group in the periodic table and go to the last element in the group and that has the highest atomic # the nitrogen family can form five covalent bonds, Group numbers tell us the number of valence electron and period number tells us.

2 in the 5s, 5 in the 5p and up to 10 in the 4d, although it only needs to use 4 of its 4d electrons to do the bonding in this molecule. Phosphorus is a group 15 element. Thus it has three shells and 5 electrons in its outermost shell.

This group of elements consists of halogens. The third shell is the outer valence shell, so it has 5 valence electrons. Group v (the nitrogen family) has five valence electrons.
 Source: slidetodoc.com
Source: slidetodoc.com
These elements include nitrogen (n), phosphorus (p), arsenic (as), antimony (sb) and bismuth (bi). Which does not follow octet rule? The electronic configuration of phosphorous is 2,8,5.
 Source: socratic.org
Source: socratic.org
2 in the 5s, 5 in the 5p and up to 10 in the 4d, although it only needs to use 4 of its 4d electrons to do the bonding in this molecule. Why can iodine have 10 electrons? Two electrons can go into first shell, eight in the second shell, and it has five more in the third shell.
 Source: wikihow.com
Source: wikihow.com
Valence electrons in hydrogen (h) 1: The electron is one of the most important factors in determining how an atom will react with another atom or. The formal charge on the oxygen atom in the hydronium ion is a.
 Source: chem.libretexts.org
Source: chem.libretexts.org
Any element in group 18 has eight valence electrons (except for helium, which has a total of just two electrons). An element has 5 valency electrons. The electrons that are held less tightly by the nucleus are the valence electrons and are available for chemical reactions.
 Source: quizlet.com
Source: quizlet.com
Thus phosphorus has 5 valence valence electrons. The electrons that are held less tightly by the nucleus are the valence electrons and are available for chemical reactions. The elements of group 15 (column) va of the periodic table all have electron configurations of s2p3 , giving them five valence electrons.
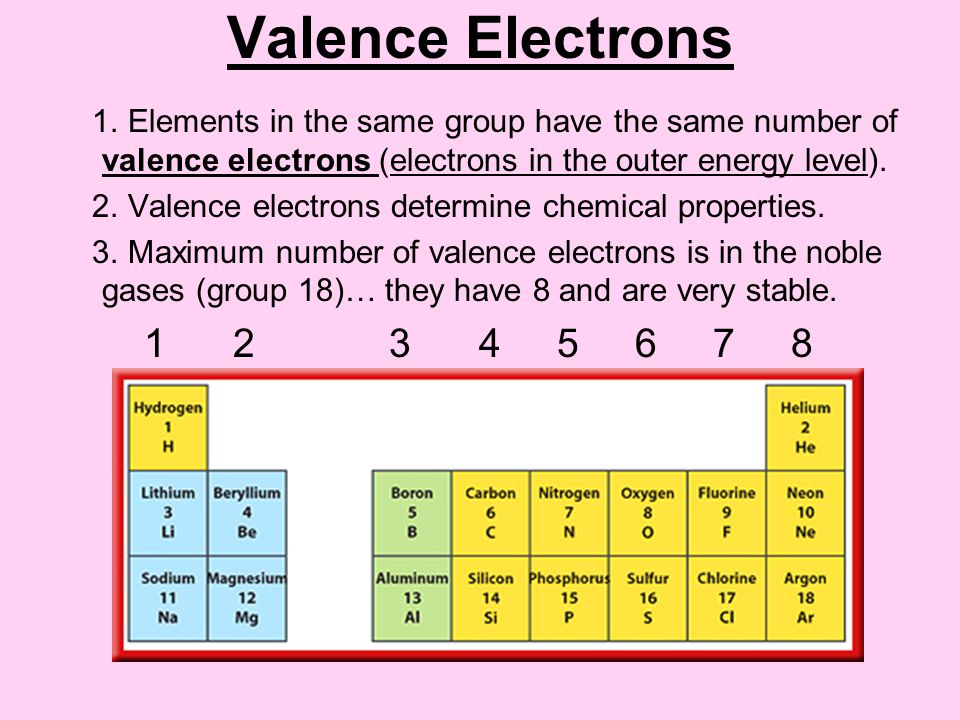 Source: slideplayer.com
Source: slideplayer.com
The elements of the group 15 (column) va of the periodic table all have electron configurations of s2p3, giving them five valence electrons. These elements include nitrogen (n), phosphorus (p), arsenic (as), antimony (sb), and bismuth (bi). The elements of the group 15 (column) va of the periodic table all have electron configurations of s2p3, giving them five valence electrons.

Source: slideplayer.com
Therefore, elements whose atoms can have the same number of valence electrons are grouped together in the periodic table of the elements. The given configuration is and this shows that there are seven valence electrons present in the outermost shell. Arsenic is in group 5 and period 4.
Also Read :






