But benzene has a higher molecular mass compared with simple alkanes such as methane, ethane. # carbons acyclic alkane # of isomers 4 butane 2 5 pentane 3 6 hexane 5 seven heptane 9 6 contents hide 1 which isomer.
Which Compound Would Have The Highest Boiling Point. B) ch 3 ch 3. If two compounds with same molecular weight then there are different factors which determine the boiling point of the organic compounds. But benzene has a higher molecular mass compared with simple alkanes such as methane, ethane. Of the diatomic elements (h2, n2, o2, f2, cl2, br2, i2), all have dispersion forces.
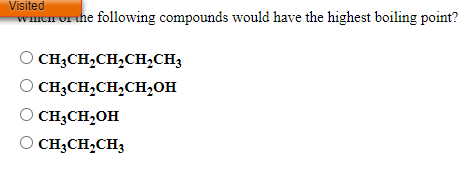 Answered: Wich Or The Following Compounds Would… | Bartleby From bartleby.com
Answered: Wich Or The Following Compounds Would… | Bartleby From bartleby.com
Related Post Answered: Wich Or The Following Compounds Would… | Bartleby :
The compound with the highest intermolecular forces will have the highest boiling point. Therefore the element with the greatest total number of electrons will have the highest boiling point (iodine) and the element with the smallest total number of electrons will have the lowest boiling point (hydrogen). B) ch 3 ch 3. # carbons acyclic alkane # of isomers 4 butane 2 5 pentane 3 6 hexane 5 seven heptane 9 6 contents hide 1 which isomer.
Boiling point (b.p.) of any organic compound depends on its molecular weight, if molecular weight increases, b.p.
Remember, the stronger the imf, the higher the boiling point or melting point recall that there are several types of intermolecular forces or imf (ranked from the strongest to the. If two compounds with same molecular weight then there are different factors which determine the boiling point of the organic compounds. First of all thanks for this a2a now come to the point c = o , carbon oxygen is doubly bonded so they are polar in nature due to their resonating structures and give rise to strong h bonding. Due to ability of making hydrogen bonds, boiling point of ethanol is high. Therefore the element with the greatest total number of electrons will have the highest boiling point (iodine) and the element with the smallest total number of electrons will have the lowest boiling point (hydrogen). Cycloalkanes have boiling points that are approximately 20 k higher than the corresponding straight chain alkane.
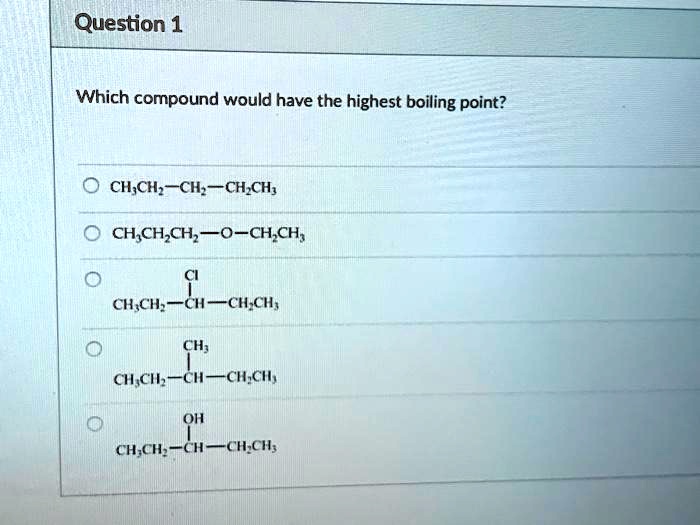 Source: numerade.com
Source: numerade.com
This is because they require more energy to be able to break the bonds during the phase transition. Because the halogens are all similar in other ways, you would expect i2 to have the greatest dispersion forces and therefore the highest boiling point (and in fact it does). The five constitutional isomers of hexane have the same molecular formula c6huh14, and the same molecular weight, 86.

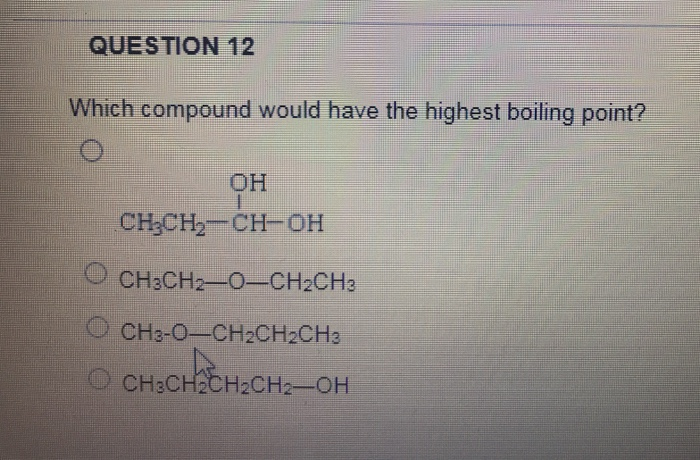
D) ch 3 ch 2 ch 2 ch 2 oh. I2 has the highest boiling point because it has the highest molar mass. If you googgled, you may find boiling point of $\ce{hf}$ is $\pu{19.5 ^{\mathrm{o}}c}$, while that of $\ce{hi}$ and $\ce{hbr}$ are $\pu{−35.4 ^{\mathrm{o}}c}$ and $\pu{−66 ^{\mathrm{o}}c}$, respectively.
 Source: oneclass.com
Source: oneclass.com
C) ch 3 ch 2 ch 2 ch 2 ch 3. # carbons acyclic alkane # of isomers 4 butane 2 5 pentane 3 6 hexane 5 seven heptane 9 6 contents hide 1 which isomer. Compounds with strong intermolecular forces have high boiling points.
![Solved] Which Compound Would Have The Highest Boiling Point? | Course Hero](https://www.coursehero.com/qa/attachment/14983820/ “Solved] Which Compound Would Have The Highest Boiling Point? | Course Hero”) Source: coursehero.com
D) ch 3 ch 2 ch 2 ch 2 oh. This is because they require more energy to be able to break the bonds during the phase transition. Boiling point (b.p.) of any organic compound depends on its molecular weight, if molecular weight increases, b.p.
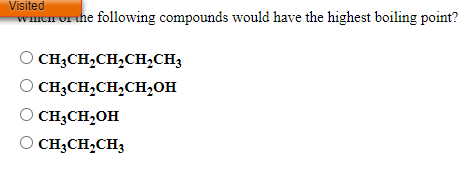 Source: bartleby.com
Source: bartleby.com
Due to strong intermolecular forces, the molecules of hf will be tightly packed in a lattice. Benzene, an aromatic organic compound which has the boiling point of 80.1 0 c. Benzene forms weak van der waals forces.
 Source: chemistry.stackexchange.com
Source: chemistry.stackexchange.com
Nacl should be the highest because nacl is an ionic compound and those boil at higher temperatures due to breaking the lattice energy of the crystal etc. Hydrogen bonding is the strongest intermolecular force. Which metal has the highest boiling point?
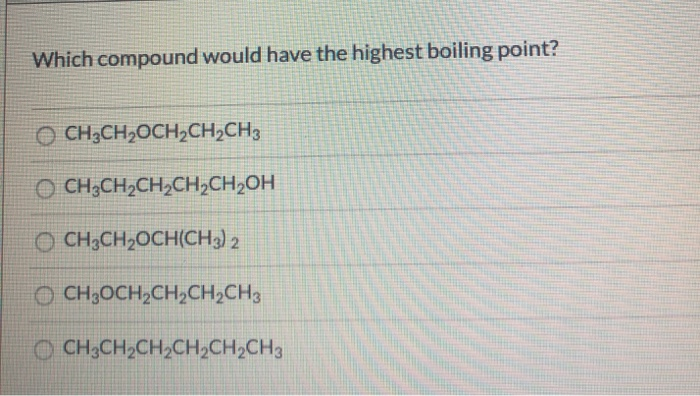 Source: chegg.com
Source: chegg.com
Typically we can rank intermolecular forces as follows: Benzene forms weak van der waals forces. Which compound has the highest boiling point quizlet?
 Source: slideplayer.com
Source: slideplayer.com
Higher molecular mass molecules have higher boiling point than the lower mass molecules. For this problem we are asked to determine which of the following compounds would have the highest boiling point. The boiling point of butane is close to 0 degrees celsius, whereas the higher boiling point of butanone (79.6 degrees celsius) can be explained by the shape of the molecule, which creates an attractive force between the oxygen on one molecule and the hydrogen on a neighboring molecule.
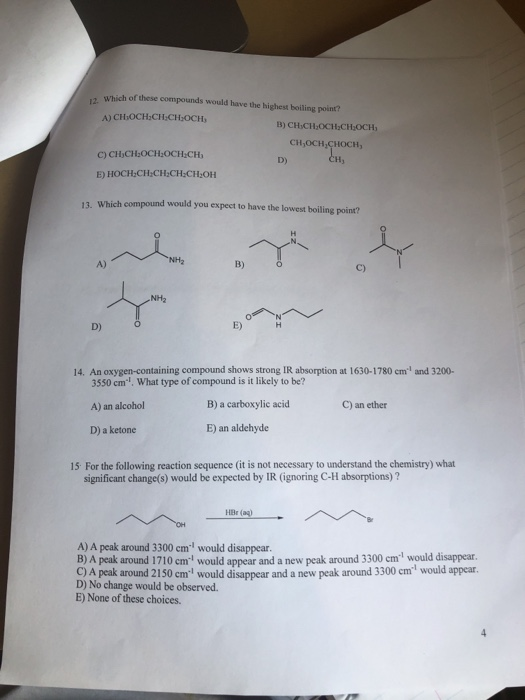 Source: chegg.com
Source: chegg.com
Due to higher molecular mass. If you googgled, you may find boiling point of $\ce{hf}$ is $\pu{19.5 ^{\mathrm{o}}c}$, while that of $\ce{hi}$ and $\ce{hbr}$ are $\pu{−35.4 ^{\mathrm{o}}c}$ and $\pu{−66 ^{\mathrm{o}}c}$, respectively. That�s why they have high boiling point than those of ester.
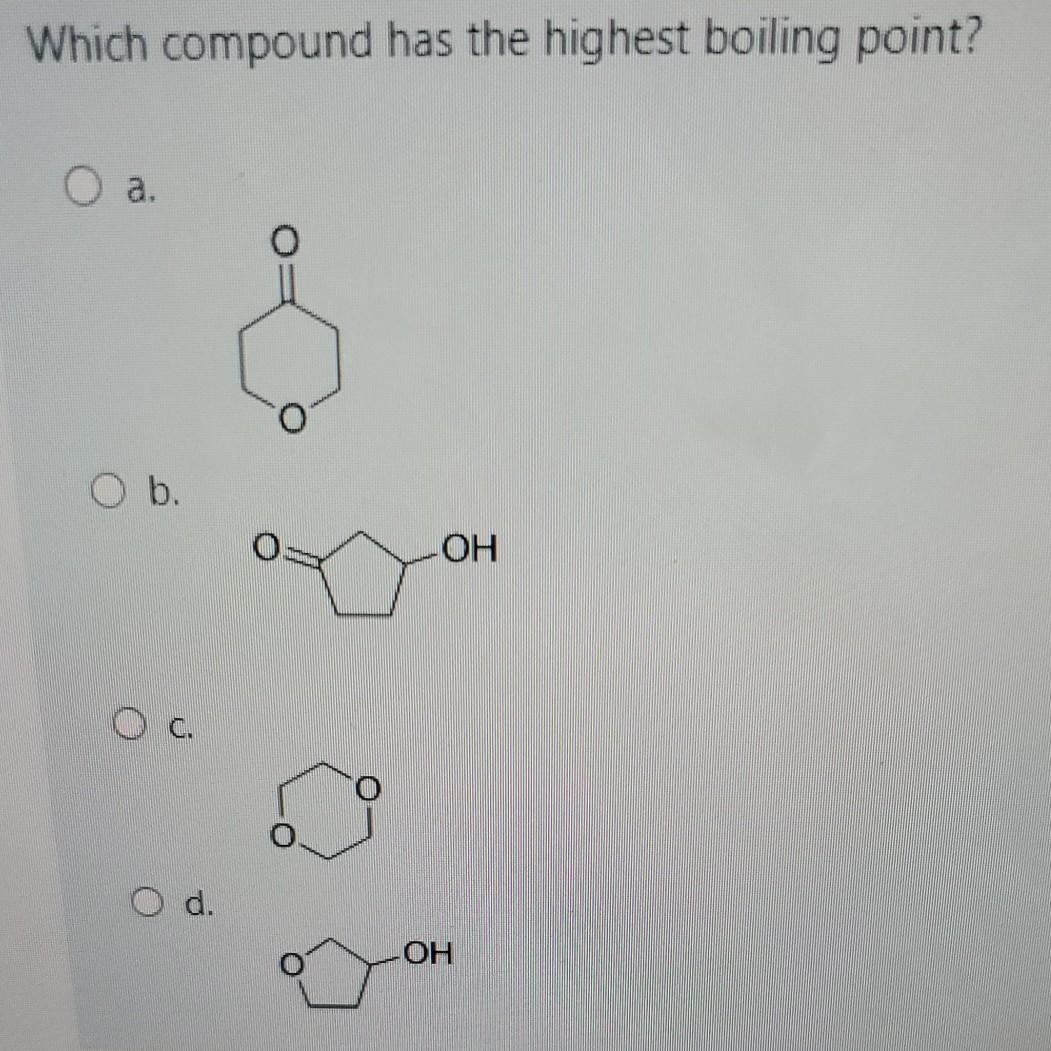 Source: bartleby.com
Source: bartleby.com
Benzene forms weak van der waals forces. The compound with the highest intermolecular forces will have the highest boiling point. If you look at the molecular structure, we have a chain of 3 carbons, then both molecules differ in number of hydrogen and oxygen atom in the compound.
 Source: studylib.net
Source: studylib.net
The compound with the highest intermolecular forces will have the highest boiling point. Due to ability of making hydrogen bonds, boiling point of ethanol is high. Of the diatomic elements (h2, n2, o2, f2, cl2, br2, i2), all have dispersion forces.
 Source: toppr.com
Source: toppr.com
Compounds with strong intermolecular forces have high boiling points. The vander waals dispersion forces increase as the length of the hydrocarbon chain increases. Due to ability of making hydrogen bonds, boiling point of ethanol is high.
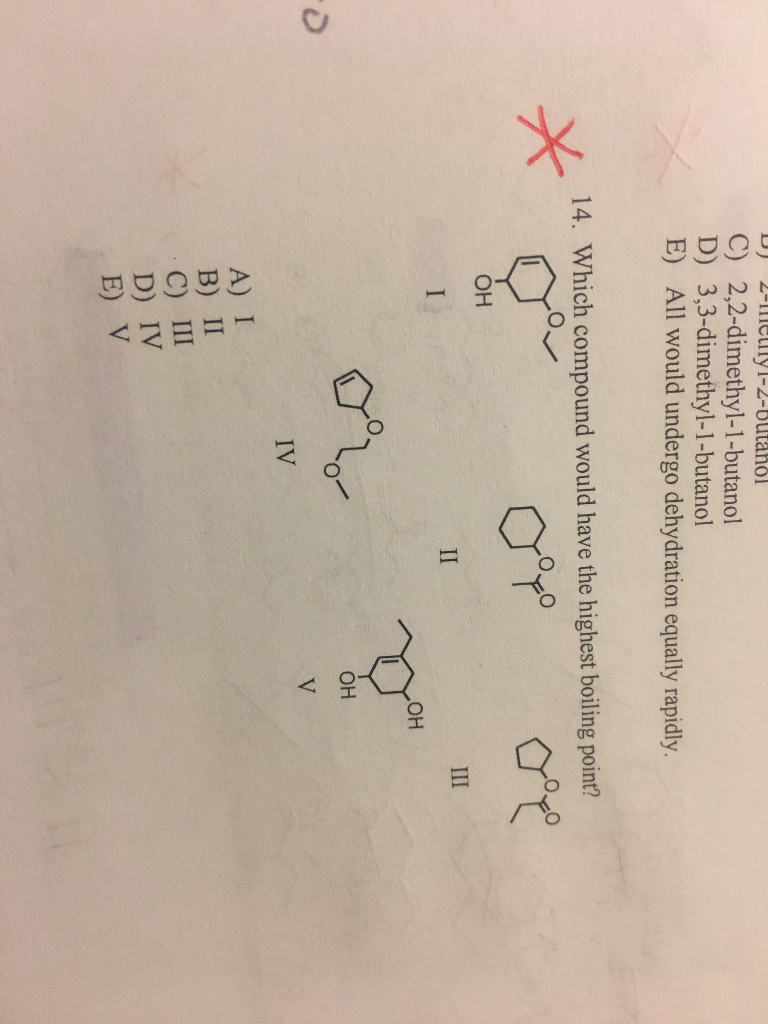 Source: chegg.com
Source: chegg.com
The compound with the highest intermolecular forces will have the highest boiling point. I2 has the highest boiling point because it has the highest molar mass. Because the halogens are all similar in other ways, you would expect i2 to have the greatest dispersion forces and therefore the highest boiling point (and in fact it does).
 Source: numerade.com
Source: numerade.com
As branching increases boiling point decreases. If two compounds with same molecular weight then there are different factors which determine the boiling point of the organic compounds. That�s why they have high boiling point than those of ester.
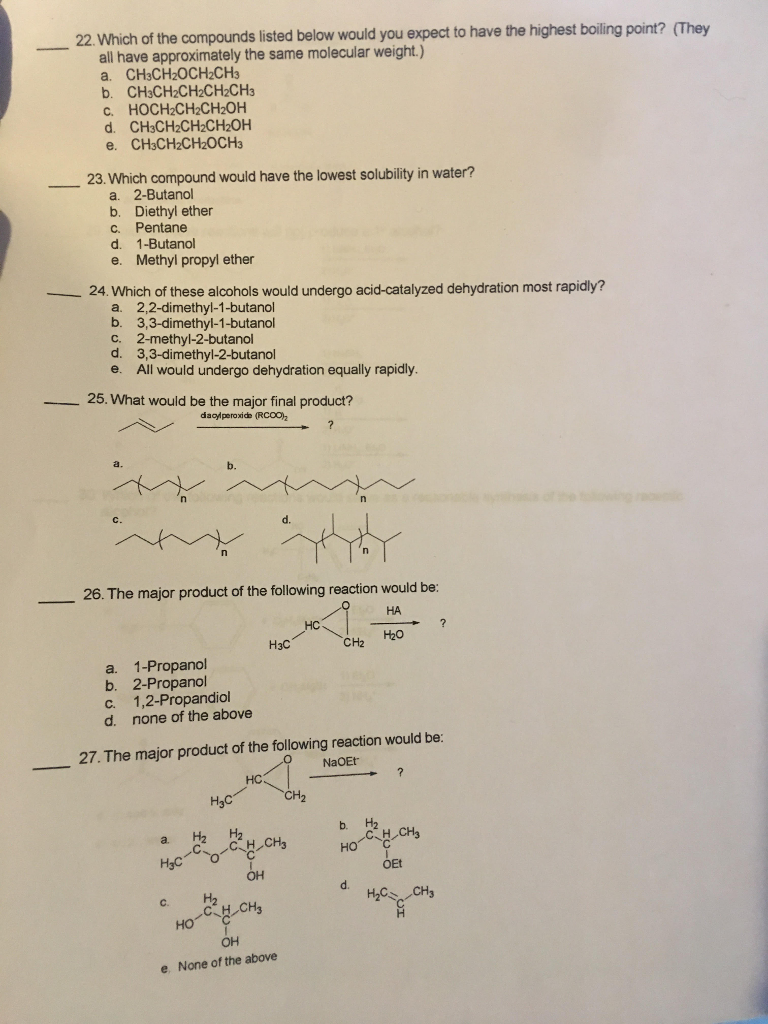 Source: chegg.com
Source: chegg.com
The simplest reason is $\ce{f}$ the highest electrnegative atom and capable of having strongest hydrogen bonding. Alcohol are found to have boiling point approximately as 78.37. The compound with the highest intermolecular forces will have the highest boiling point.
 Source: chegg.com
Source: chegg.com
Now both compounds do have hydrogen bonding because h attachment to a high electronegative element, which means boiling point is. Which compound has the highest boiling point? 1 m alc l 3 solution will have the highest boiling point is because colligative properties such as elevation in boiling point is directly proportional to number of particles in solutions and alc l 3 produces highest number of particles or ions.
 Source: chegg.com
Source: chegg.com
B) ch 3 ch 3. If you googgled, you may find boiling point of $\ce{hf}$ is $\pu{19.5 ^{\mathrm{o}}c}$, while that of $\ce{hi}$ and $\ce{hbr}$ are $\pu{−35.4 ^{\mathrm{o}}c}$ and $\pu{−66 ^{\mathrm{o}}c}$, respectively. How can you tell that water has a simple molecular structure?
 Source: clutchprep.com
Source: clutchprep.com
Cycloalkanes have boiling points that are approximately 20 k higher than the corresponding straight chain alkane. For this problem we are asked to determine which of the following compounds would have the highest boiling point. I2 has the highest boiling point because it has the highest molar mass.
 Source: clutchprep.com
Source: clutchprep.com
D) ch 3 ch 2 ch 2 ch 2 oh. First of all thanks for this a2a now come to the point c = o , carbon oxygen is doubly bonded so they are polar in nature due to their resonating structures and give rise to strong h bonding. The five constitutional isomers of hexane have the same molecular formula c6huh14, and the same molecular weight, 86.
 Source: oneclass.com
Source: oneclass.com
Higher molecular mass molecules have higher boiling point than the lower mass molecules. But benzene has a higher molecular mass compared with simple alkanes such as methane, ethane. Of the diatomic elements (h2, n2, o2, f2, cl2, br2, i2), all have dispersion forces.
Also Read :





