The alcohols are soluble in water due to the presence of the hydroxyl group in them. Hence more h bonding leads to more solubility in water.
Which Compound Should Be The Most Soluble In Water. Why c2h5oh is soluble in water? Hence, it is most soluble in water. How do you know which compound is most soluble? Ethylene glycol has two hydroxy groups both of which form hydrogen bonds with water.
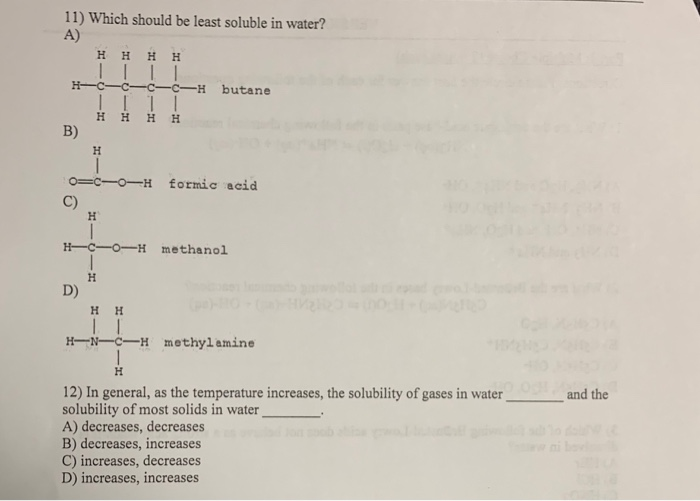 Solved 11) Which Should Be Least Soluble In Water? A) Н H- | Chegg.com From chegg.com
Solved 11) Which Should Be Least Soluble In Water? A) Н H- | Chegg.com From chegg.com
Related Post Solved 11) Which Should Be Least Soluble In Water? A) Н H- | Chegg.com :
Which compound would be the most soluble in water? View available hint(s) ketones aldehydes alcohols. Because of its high polarity, water is the most common solvent for ionic compounds. As a triol, it would be quite thick and syrupy, and probably would dissolve in water in any proportion.
Which compound is the most soluble in water?a.
Ethylene glycol has two hydroxy groups both of which form hydrogen bonds with water. Ethylene glycol has two hydroxy groups both of which form. Which silver halide has the least solubility in water? Ionic substances are generally most soluble in polar solvents; So what do i mean by a molecule being polar or non polar, for example let’s take a water molecule h2o. For example, benzoic acid is not soluble in water, yet it is soluble in sodium hydroxide solution
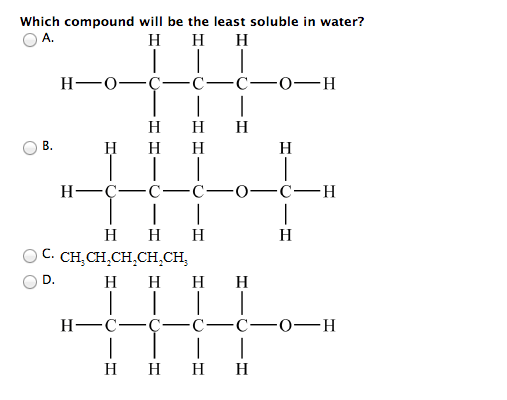 Source: chegg.com
Source: chegg.com
Pbso 4 is poorly soluble. Ethylene glycol has two hydroxy groups both of which form hydrogen bonds with water. Ethylene glycol is most soluble in water.
 Source: chegg.com
Source: chegg.com
Ionic substances are generally most soluble in polar solvents; As examples, ethanoic acid is soluble in water. Solved which compound should be the most soluble in water?
 Source: chegg.com
Source: chegg.com
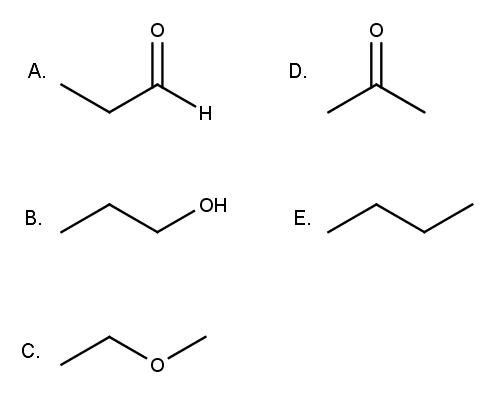
Subsequently, one may also ask, which compounds are most soluble in water? A) carbonyl b) ether c) alkyl d) aromatic e) hydroxyl. Which compound would be the most soluble in water?
![Solved] How To Determine Most Soluble In Water? | Course Hero](https://www.coursehero.com/qa/attachment/15331942/ “Solved] How To Determine Most Soluble In Water? | Course Hero”) Source: coursehero.com
A) carbonyl b) ether c) alkyl d) aromatic e) hydroxyl. But, when those compound�s molecular mass increases, solubility in water is decreased. Exceptions include hydroxide salts of.
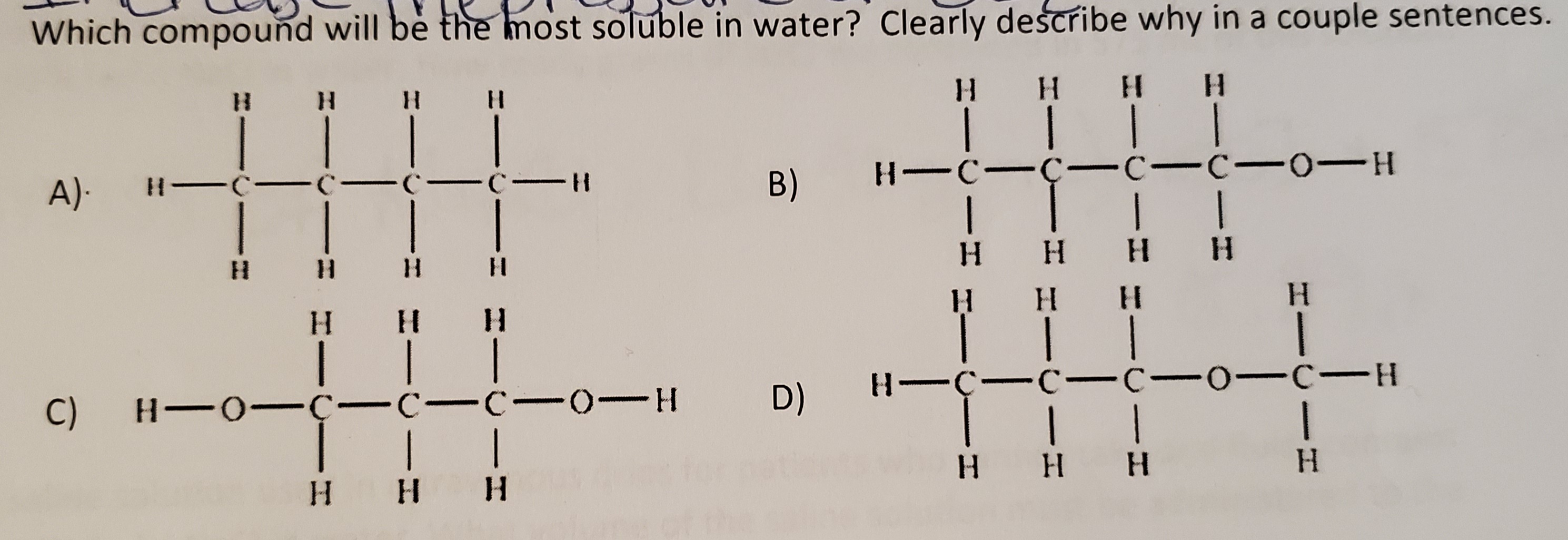 Source: bartleby.com
Source: bartleby.com
The electro negativity of oxygen is greater than that. Here you have to consider whether that molecule is polar or non polar. Because of its high polarity, water is the most common solvent for ionic compounds.
 Source: clutchprep.com
Source: clutchprep.com
What makes a compound more soluble in water? A) carbonyl b) ether c) alkyl d) aromatic e) hydroxyl. Ethylene glycol is most soluble in water.
 Source: quizlet.com
Source: quizlet.com
Ethylene glycol has two hydroxy groups both of which form. Which of the following compound is most soluble in water? Which group is the most soluble in water (assuming masses and number of carbons are equivalent)?
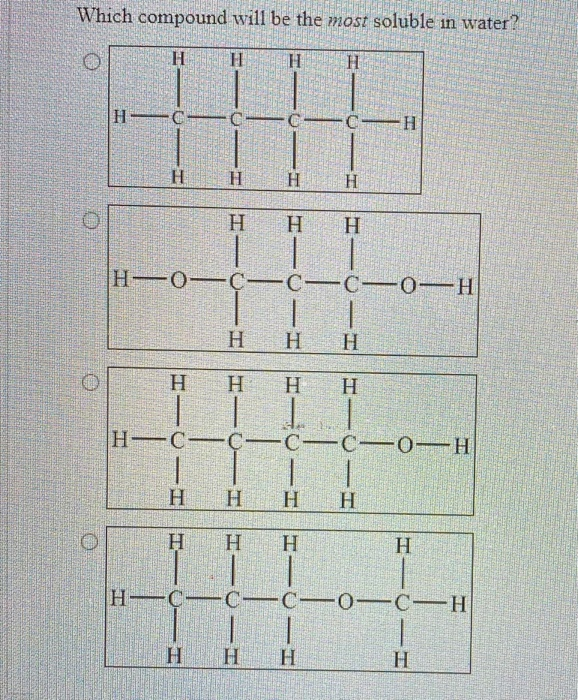 Source: chegg.com
Source: chegg.com
These solubility results indicate that vitamin d is most likely a nonpolar compound. Which of the following compound is most soluble in water? Diethyl ether and ethyl benzoate do not form hydrogen bonds with water.
 Source: youtube.com
Source: youtube.com
So for this, we need to draw the lewis structures of the given choices and determine their polarity. Diethyl ether and ethyl benzoate do not form hydrogen bonds with water. It depends on the solution and the solute.
 Source: quizlet.com
Source: quizlet.com
A) ch3ch2ch2ch2ch2ch2ch2ch3 b) ch3ch2ch3 c) ch3ch2ch2ch3 d) ch3ch2ch2ch2oh e) ch3ch2ch2oh. Ethylene glycol has two hydroxy groups both of which form. Exceptions include hydroxide salts of.
 Source: clutchprep.com
Source: clutchprep.com
So what do i mean by a molecule being polar or non polar, for example let’s take a water molecule h2o. Lead halides are, however, soluble in hot water. Which compound is the most soluble in water?a.
 Source: clutchprep.com
Source: clutchprep.com
An “emulsifying agent” is a compound that helps stabilizea hydrophobic colloid in a hydrophilic solv. Greater is the number of hydrogen bonds, greater is the extent of hydrogen bonding and greater is the solubility in water. Ethylene glycol has two hydroxy groups both of which form hydrogen bonds with water.
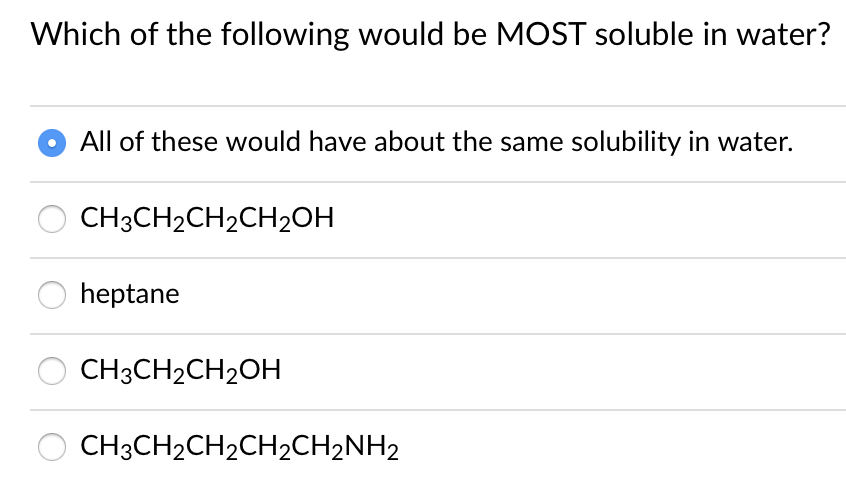 Source: bartleby.com
Source: bartleby.com
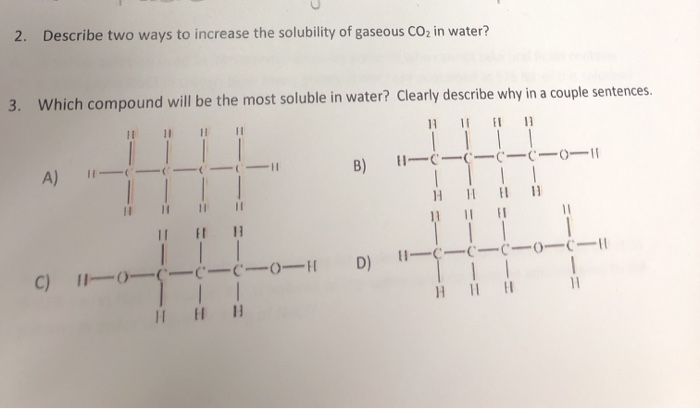
Because of its high polarity, water is the most common solvent for ionic compounds. The next most soluble would be 4 (a diol), then 1 (an alcohol) and finally 3 would be completely insoluble in water. Which compound in each pair is more soluble in water?
 Source: youtube.com
Source: youtube.com
Among given compounds, ethylene glycol ( ho−ch2−ch2−oh ) is the most soluble in water. As a triol, it would be quite thick and syrupy, and probably would dissolve in water in any proportion. But the solubility decreases with increase in the carbon chain length as it hinders the bonding of hydroxyl with water.

A) ch3ch2ch2ch2ch2ch2ch2ch3 b) ch3ch2ch3 c) ch3ch2ch2ch3 d) ch3ch2ch2ch2oh e) ch3ch2ch2oh. What organic molecules are soluble in water? Also it has a bulky hydrophobic phenyl group.
 Source: clutchprep.com
Source: clutchprep.com
Which silver halide has the least solubility in water? Exceptions include hydroxide salts of. Determine the central atom in this molecule.
 Source: chegg.com
Source: chegg.com
Which compound in each of the following pairs would be more soluble in water? Which of the following compound is most soluble in water? Halides are group 7 elements.
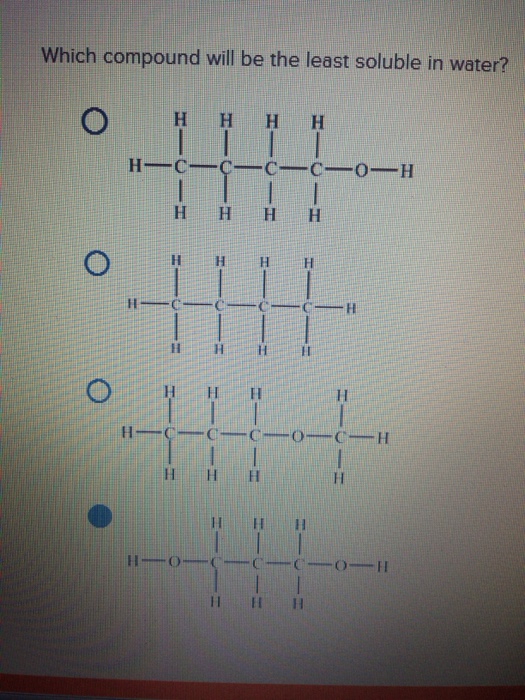
It forms most extensive intermolecular hydrogen bonds with water. Emulsifying agent is defined as the substance that helps in the formation of emulsion. Which silver halide has the least solubility in water?
 Source: reddit.com
Source: reddit.com
Vitamin d is tested for its solubility in water and benzene (c6h6), and is found to be insoluble in water and soluble in benzene. As examples, ethanoic acid is soluble in water. The number of hydrogen bonds is higher, the hydrogen bonding strength is greater and the water solubility is also high.
 Source: clutchprep.com
Source: clutchprep.com
Which group is the most soluble in water (assuming masses and number of carbons are equivalent)? Also it has a bulky hydrophobic phenyl group. The higher the lattice energy, the more polar the solvent must be to overcome the lattice energy and dissolve the substance.
Also Read :





