Which compound is soluble in water and why? Among given compounds, ethylene glycol ( h o − c h 2 − c h 2 − o h ) is the most soluble in water.
Which Compound Is The Most Soluble In Water. What compounds are most soluble in water? Among given compounds, ethylene glycol ( ho−ch2−ch2−oh ) is the most soluble in water. Hence more h bonding leads to more solubility in water. Among given compounds, ethylene glycol ( h o − c h 2 − c h 2 − o h ) is the most soluble in water.

Related Post Solved Which Compound Is The Most Soluble In Water? O | Chegg.com :
The value of the constant identifies the degree to which the compound can dissociate in water. This value rises with heating, and is the only. Among given compounds, ethylene glycol ( ho−ch2−ch2−oh ) is the most soluble in water. The solubility product constant describes the equilibrium between a solid and its constituent ions in a solution.
Greater is the number of hydrogen bonds, greater is the extent of hydrogen bonding and greater is the solubility in water.
The common salt is polar in nature and it is soluble in water. Recall that the main idea in dissolution is like dissolves like, which means compounds with the same polarity and intermolecular force can dissolve each other. Which compound is soluble in cold water? Reset help least soluble in water most soluble in water octanoic acid methanolo acid (oric acid) pentanoic acid the correct ranking cannot be determined previous question next question company But when alkyl group gets larger, solubility decreases. Ethylene glycol has two hydroxy groups both of which form hydrogen bonds with water.
 Source: oneclass.com
Source: oneclass.com
The sodium (na) has a positive charge and the chloride (cl) has a negative charge. Among given compounds, ethylene glycol ( ho−ch2−ch2−oh ) is the most soluble in water. Which carbonates are soluble in water?
 Source: youtube.com
Source: youtube.com
The solubility product constant describes the equilibrium between a solid and its constituent ions in a solution. Greater is the number of hydrogen bonds, greater is the extent of hydrogen bonding and greater is the solubility in water. For example, potassium carbonate (k2co3), sodium carbonate (na2co3), and all other group 2 carbonates are soluble in water except lithium carbonate.
 Source: stringfixer.com
Source: stringfixer.com
Hence more h bonding leads to more solubility in water. Hydroxides of sodium, potassium and ammonium are soluble in water. Remember that, carboxylic acids dissolve.
 Source: clutchprep.com
Source: clutchprep.com
Ethylene glycol has two hydroxy groups both of which form hydrogen bonds with water. When naoh is administered, it dissolves in the blood, and thus potentially has side effects far greater than its advantageous solubility in the testing bottle. Hydroxides of sodium, potassium and ammonium are soluble in water thus sodium hydroxide(naoh), potassium hydroxide (koh) and ammonium hydroxide.
 Source: reddit.com
Source: reddit.com
Among the given compounds, sugar is the most soluble compound in water. Most metal carbonates do not dissolve in water, with a few exceptions while most alkaline metal carbonates are soluble in water. The value of the constant identifies the degree to which the compound can dissociate in water.
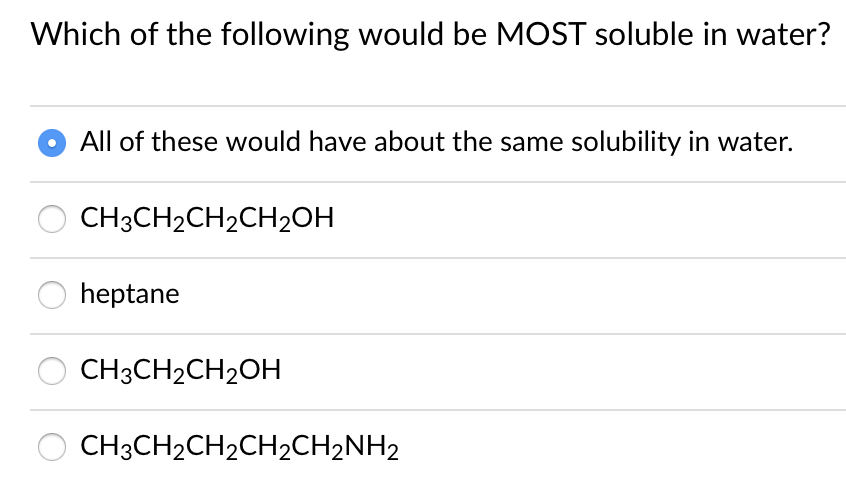 Source: bartleby.com
Source: bartleby.com
Ethylene glycol has two hydroxy groups both of which form hydrogen bonds with water. Which carbonates are soluble in water? Apparently 300 g of each compound will dissolve in 40 g of water without saturating it room temperature , giving a lower bound to their combined solubility of 15 000 g/kg water.
 Source: transtutors.com
Source: transtutors.com
Lead chloride is also insoluble in cold water but is soluble in hot water. Ethylene glycol has two hydroxy groups both of which form hydrogen bonds with water. A polar molecule is one that�s neutral, or uncharged, but has an asymmetric internal distribution of.
 Source: bartleby.com
Source: bartleby.com
Hydroxides of sodium, potassium and ammonium are soluble in water thus sodium hydroxide(naoh), potassium hydroxide (koh) and ammonium hydroxide. Choose the compound that is the most soluble in water. Remember that, carboxylic acids dissolve.
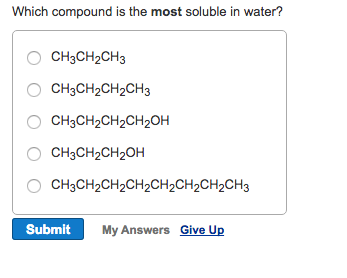 Source: chegg.com
Source: chegg.com
Why c2h5oh is soluble in water? Ethylene glycol is most soluble in water. We’re being asked to determine the compound that is most soluble in water.

Among given compounds, ethylene glycol ( ho−ch2−ch2−oh ) is the most soluble in water. Greater is the number of hydrogen bonds, greater is the extent of hydrogen bonding and greater is the solubility in water. Apparently 300 g of each compound will dissolve in 40 g of water without saturating it room temperature , giving a lower bound to their combined solubility of 15 000 g/kg water.
 Source: clutchprep.com
Source: clutchprep.com
Ethylene glycol has two hydroxy groups both of which form hydrogen bonds with water. Sodium carbonate (na 2 co 3),potassium carbonate (k 2 co 3) and ammonium carbonate (nh 4) 2 co 3 are soluble in water. Because sugar has six hydroxyl groups.
![Solved] How To Determine Most Soluble In Water? | Course Hero](https://www.coursehero.com/qa/attachment/15331942/ “Solved] How To Determine Most Soluble In Water? | Course Hero”) Source: coursehero.com
Complete step by step solution: This value rises with heating, and is the only. Usually, they dissolve in warm and hot water very quickly.

Hence, option (c) is incorrect. In other words, ethanol is soluble in water because it is a polar solvent. But when alkyl group gets larger, solubility decreases.
![Solved] Which Of The Following Compounds Would Be The Most Soluble In Water? | Course Hero](https://www.coursehero.com/qa/attachment/15926613/ “Solved] Which Of The Following Compounds Would Be The Most Soluble In Water? | Course Hero”) Source: coursehero.com
Ethylene glycol has two hydroxy groups both of which form hydrogen bonds with water. Among the following compounds, the strontium fluoride has the least solubility. Circle the compound below that would be most soluble in water.
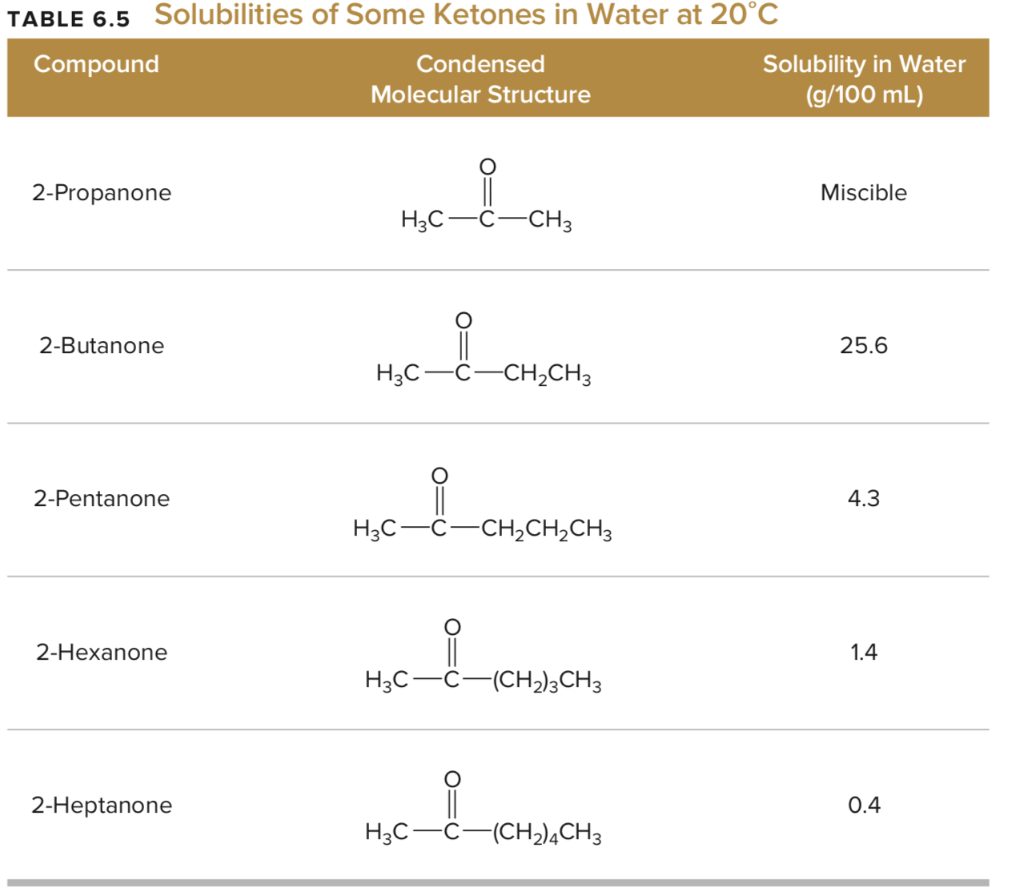 Source: chegg.com
Source: chegg.com
Reset help least soluble in water most soluble in water octanoic acid methanolo acid (oric acid) pentanoic acid the correct ranking cannot be determined previous question next question company But when alkyl group gets larger, solubility decreases. This value rises with heating, and is the only.
 Source: transtutors.com
Source: transtutors.com
In other words, ethanol is soluble in water because it is a polar solvent. Which group is the most soluble in water (assuming masses and number of carbons are equivalent)? Lead sulfate is insoluble in cold water whereas most of the sulfates are soluble in cold water.
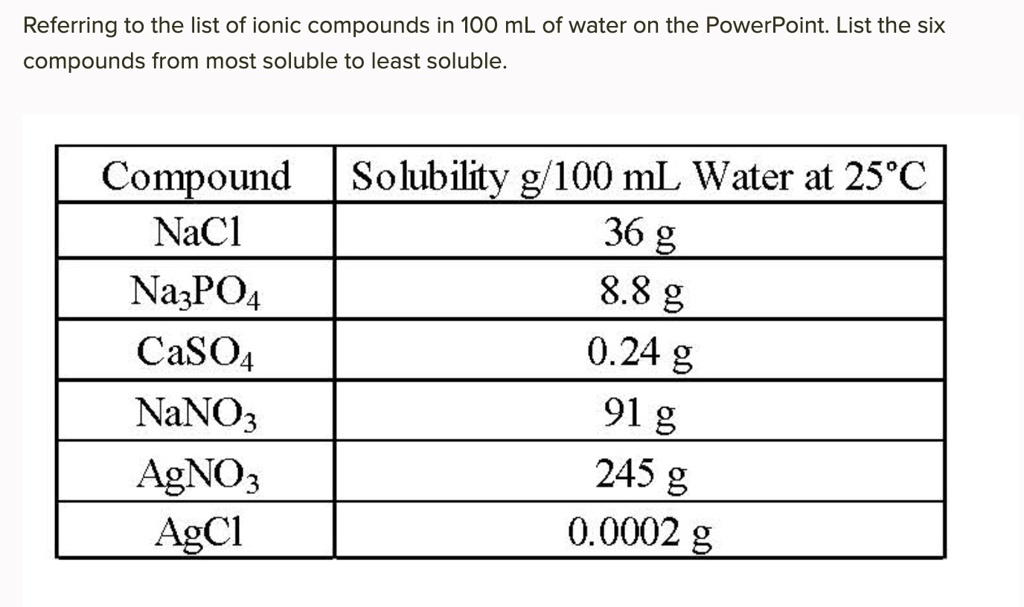 Source: numerade.com
Source: numerade.com
Apparently 300 g of each compound will dissolve in 40 g of water without saturating it room temperature , giving a lower bound to their combined solubility of 15 000 g/kg water. In the context of pharmaceuticals, naoh is soluble in water, yet this does not necessarily mean that the compound is soluble in the blood stream or that it is even safe. Greater is the number of hydrogen bonds, greater is the extent of hydrogen bonding and greater is the solubility in water.
 Source: quizlet.com
Source: quizlet.com
Among given compounds, ethylene glycol ( ho−ch2−ch2−oh ) is the most soluble in water. Water is a polar molecule that exhibits hydrogen bonding, so the best solute is another polar compound. Circle the compound below that would be most soluble in water.
 Source: youtube.com
Source: youtube.com
But when alkyl group gets larger, solubility decreases. The value of the constant identifies the degree to which the compound can dissociate in water. Water is a polar molecule that exhibits hydrogen bonding, so the best solute is another polar compound.
 Source: clutchprep.com
Source: clutchprep.com
Hence more h bonding leads to more solubility in water. Remember that, carboxylic acids dissolve. * water is sometimes called the universal solvent. * water is good at dissolving ions and polar molecules, but poor at dissolving nonpolar molecules.
Also Read :





