These all have h as the first element in the formula. This 14 words question was answered by heather l.
Which Compound Is An Arrhenius Acid. Which of the following in an arrhenius acid? A) h 2 so 4. An arrhenius acid is a compound, which ionizes to yield hydrogen ions (h + ) in aqueous solution. Only hydrogen atoms that are part of a highly polar covalent bond are ionizable.
 1 Acids And Bases Definition Of Acids Arrhenius Acid: A Substance That Releases H + In Water ( E.g. Hcl) H + + H 2 O H 3 O + Hydronium. - Ppt Download From slideplayer.com
1 Acids And Bases Definition Of Acids Arrhenius Acid: A Substance That Releases H + In Water ( E.g. Hcl) H + + H 2 O H 3 O + Hydronium. - Ppt Download From slideplayer.com
Related Post 1 Acids And Bases Definition Of Acids Arrhenius Acid: A Substance That Releases H + In Water ( E.g. Hcl) H + + H 2 O H 3 O + Hydronium. - Ppt Download :
The chemical formulas of arrhenius acids are written with the acidic hydrogens first. An arrhenius acid is a compound that increases the h + ion concentration in aqueous solution. The chemical formula of vinegar is {eq}ch_{3}cooh, {/eq}. An arrhenius base is a compound that increases the oh − ion concentration in aqueous solution.
An arrhenius acid is a compound that increases the h + ion concentration in aqueous solution.
In other words, it increases the number of h+ ions in the water. The h+ ion produced by an arrhenius acid is always associated with a water molecule to form the hydronium ion. Acetic acid is a carboxylic acid. Only hydrogen atoms that are part of a highly polar covalent bond are ionizable. As a compound that increases the concentration of hydrogen ion (h +) in aqueous solution. This theory states a compound is classified as an acid when it donates the proton to other species and itself forms a conjugate base.
 Source: slidetodoc.com
Source: slidetodoc.com
The chemical formulas of arrhenius acids are written with the acidic hydrogens first. According to him, an acid is a compound which can readily give up protons or hydrogen ion in aqueous or water solution. Is a compound that increases the h + ion concentration in aqueous solution.
 Source: clutchprep.com
Source: clutchprep.com
An arrhenius acid is a substance that dissociates in water to form hydrogen ions (h+). An arrhenius acid is a compound that increases the h + ion concentration in aqueous solution. The reaction between an arrhenius acid and an arrhenius base is called neutralization and results in the formation of water and a salt.
 Source: slideplayer.com
Source: slideplayer.com
What is the arrhenius definition of an acid of a base quizlet? An arrhenius acid is a substance that when added to water increases the concentration of h+ ions present.hcl is an example of an arrhenius acid and, for example, naoh is an example of an arrhenius base. An arrhenius base is any species that increases the concentration of in aqueous solution.

Among the four options, (1) h2so4 is the correct one because when it dissolves in water, it liberates h+. What is the arrhenius definition of an acid of a base quizlet? The first compound under investigation is vinegar.
 Source: clutchprep.com
Source: clutchprep.com
Bases are compounds that ionize to. Arrhenius base = koh, lioh, ba (oh) 2 , zn (oh) 2. These all have h as the first element in the formula.
 Source: clutchprep.com
Source: clutchprep.com
According to arrhenius theory of acids and bases, an arrhenius acid produces h+ when dissolved in water. These all have h as the first element in the formula. The chemical formula of vinegar is {eq}ch_{3}cooh, {/eq}.
 Source: chegg.com
Source: chegg.com
Hence, we can say the hclo 4 compound is an arrhenius acid compound. An arrhenius acid is a compound that increases the h + ion concentration in aqueous solution. According to arrhenius theory of acids and bases, an arrhenius acid produces h+ when dissolved in water.
 Source: study.com
Source: study.com
Is a compound that increases the h + ion concentration in aqueous solution. The h+ ion produced by an arrhenius acid is always associated with a water molecule to form the hydronium ion. The chemical formulas of arrhenius acids are written with the acidic hydrogens first.
 Source: slideplayer.com
Source: slideplayer.com
According to this, a compound is an acid when it furnishes an h + ion on dissolving in an aqueous solution or increases the concentration of hydrogen ion in the solution. In the following choices of compounds, hcl or hydrogen chloride or hydrochloric acid is an example of arrhenius acid. An arrhenius acid is a substance that dissociates in water to form hydrogen ions (h+).
 Source: chegg.com
Source: chegg.com
Among the four options, (1) h2so4 is the correct one because when it dissolves in water, it liberates h+. In aqueous solution, ions immediately react with water molecules to form hydronium ions,. Many acids are simple compounds that release a hydrogen cation into solution when they dissolve.
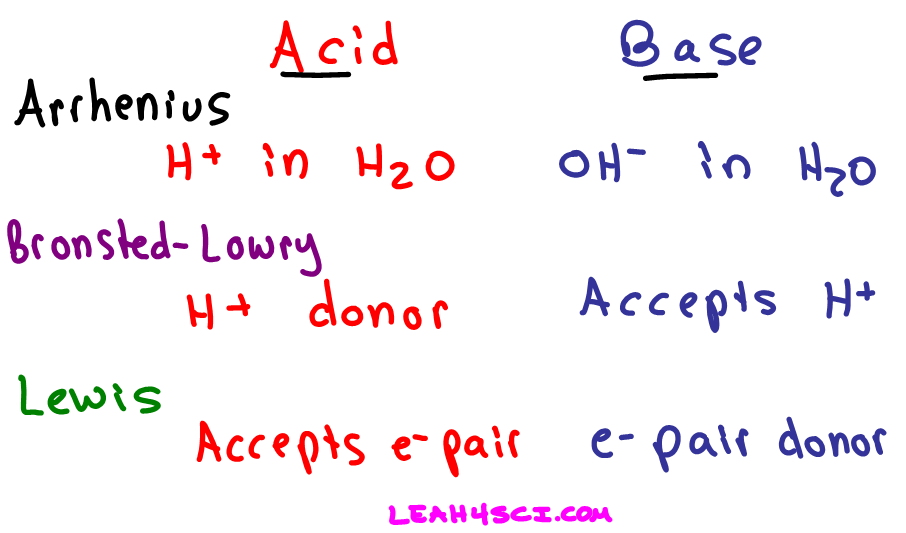 Source: leah4sci.com
Source: leah4sci.com
These all have h as the first element in the formula. Learn this topic by watching arrhenius acid and base concept videos. An arrhenius base is a compound that increases the oh − ion concentration in aqueous solution.
 Source: flexbooks.ck12.org
Source: flexbooks.ck12.org
Acids are molecular compounds with ionizable hydrogen atoms. These all have h as the first element in the formula. An arrhenius acid is a compound, which ionizes to yield hydrogen ions (h + ) in aqueous solution.
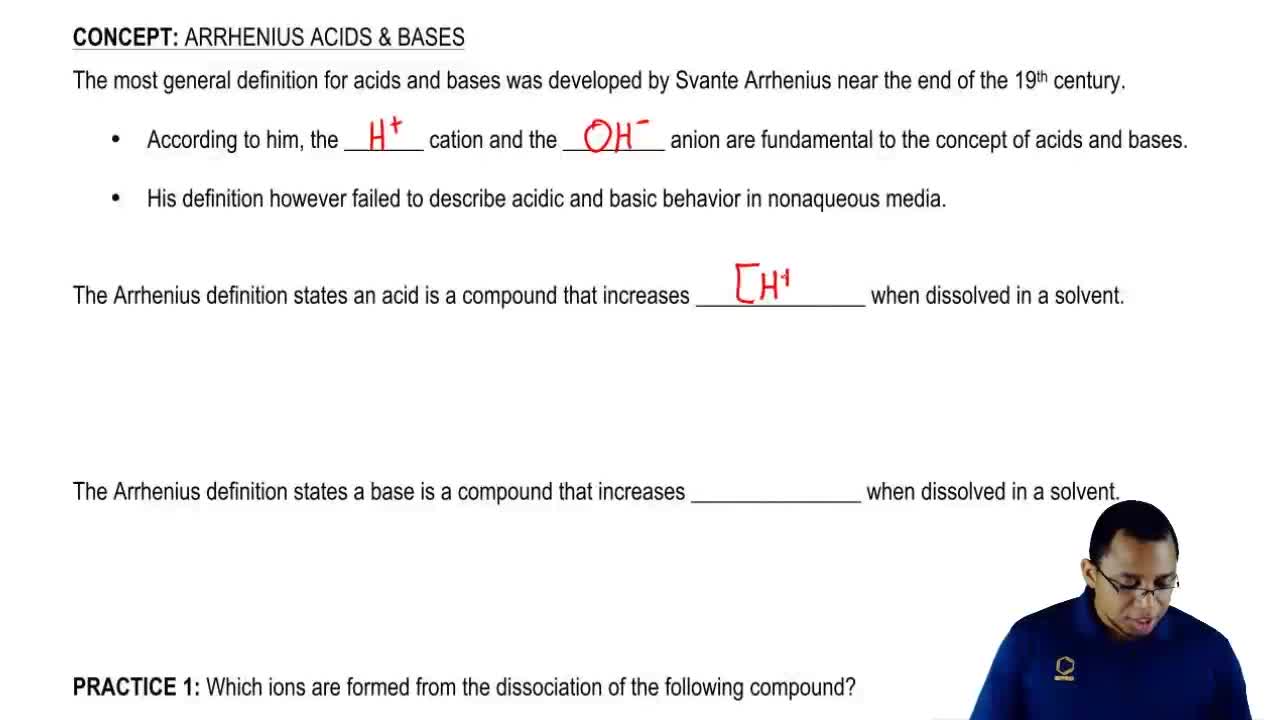 Source: clutchprep.com
Source: clutchprep.com
The reaction between an arrhenius acid and an arrhenius base is called neutralization and results in the formation of water and a salt. According to this, a compound is an acid when it furnishes an h + ion on dissolving in an aqueous solution or increases the concentration of hydrogen ion in the solution. Learn this topic by watching arrhenius acid and base concept videos.

C) nh 2 ch 3. When you take organic chemistry, you will learn that carboxylic acids are usually written with the acidic h at the end. Which of the following in an arrhenius acid?
 Source: sciencenotes.org
Source: sciencenotes.org
E) more than one of these is an arrhenius acid. The h+ ion produced by an arrhenius acid is always associated with a water molecule to form the hydronium ion. E) more than one of these is an arrhenius acid.
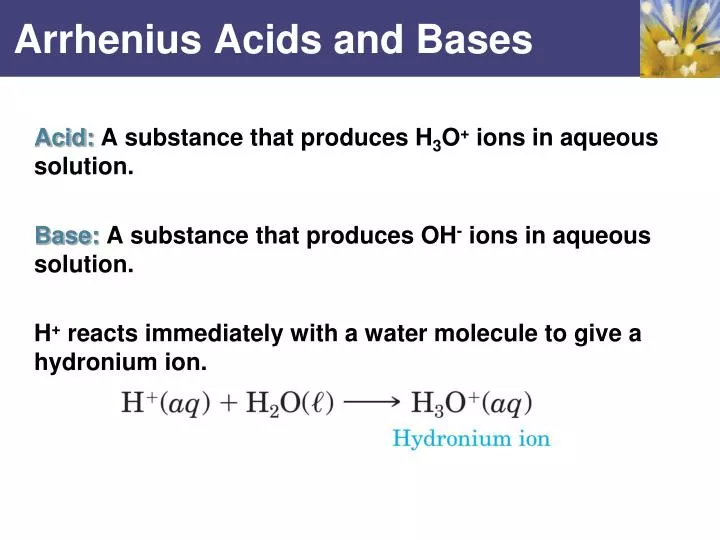 Source: slideserve.com
Source: slideserve.com
Acid is a compound that dissolves in water releasing h + ions. An arrhenius acid is a substance that when added to water increases the concentration of h+ ions present.hcl is an example of an arrhenius acid and, for example, naoh is an example of an arrhenius base. An arrhenius acid is a compound, which ionizes to yield hydrogen ions (h + ) in aqueous solution.
 Source: study.com
Source: study.com
In other words, it increases the number of h+ ions in the water. Among the four options, (1) h2so4 is the correct one because when it dissolves in water, it liberates h+. E) more than one of these is an arrhenius acid.
 Source: chegg.com
Source: chegg.com
What is the arrhenius definition of an acid of a base quizlet? In other words, it increases the number of h+ ions in the water. An arrhenius acid a compound that increases the hydrogen ion concentration in aqueous solution.
 Source: study.com
Source: study.com
Among the four options, (1) h2so4 is the correct one because when it dissolves in water, it liberates h+. The chemical formula of vinegar is {eq}ch_{3}cooh, {/eq}. When you take organic chemistry, you will learn that carboxylic acids are usually written with the acidic h at the end.
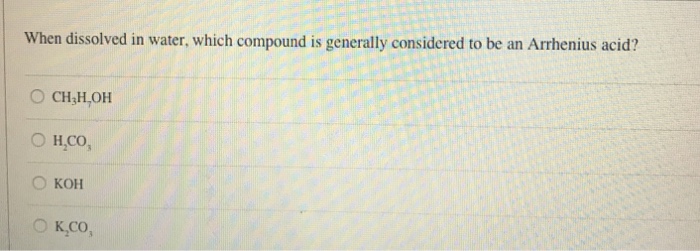
Arrhenius acid and base definition. Arrhenius defined an acid a compound that increases the concentration of hydrogen ion (h +) in aqueous solution. A swedish scientist svante arrhenius, in the year 1884, proposed acid and base as the two classifications of compounds.
Also Read :





