2.the density of the solution. Sodium chloride is an ionic compound that is made up of the sodium cation and the chloride anion.
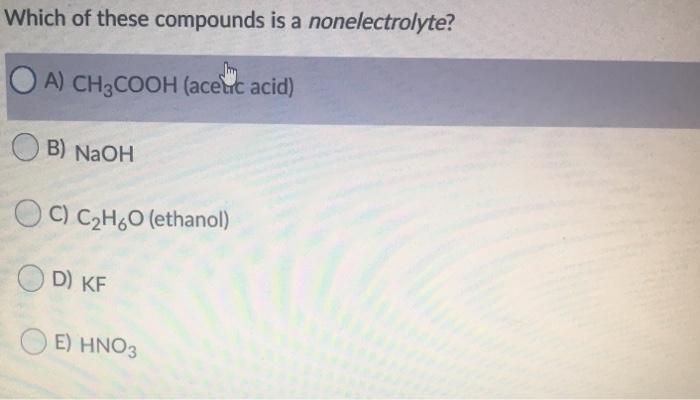
Which Compound Is A Nonelectrolyte. Is acetic acid a nonelectrolyte? Nacl (solution) is an electrolyte in the molten. For example, carbon tetrachloride ccl4 when dissolved in water does not dissociate into ions and therefore, it does not conduct electricity. They share electron pairs to form such bonds.
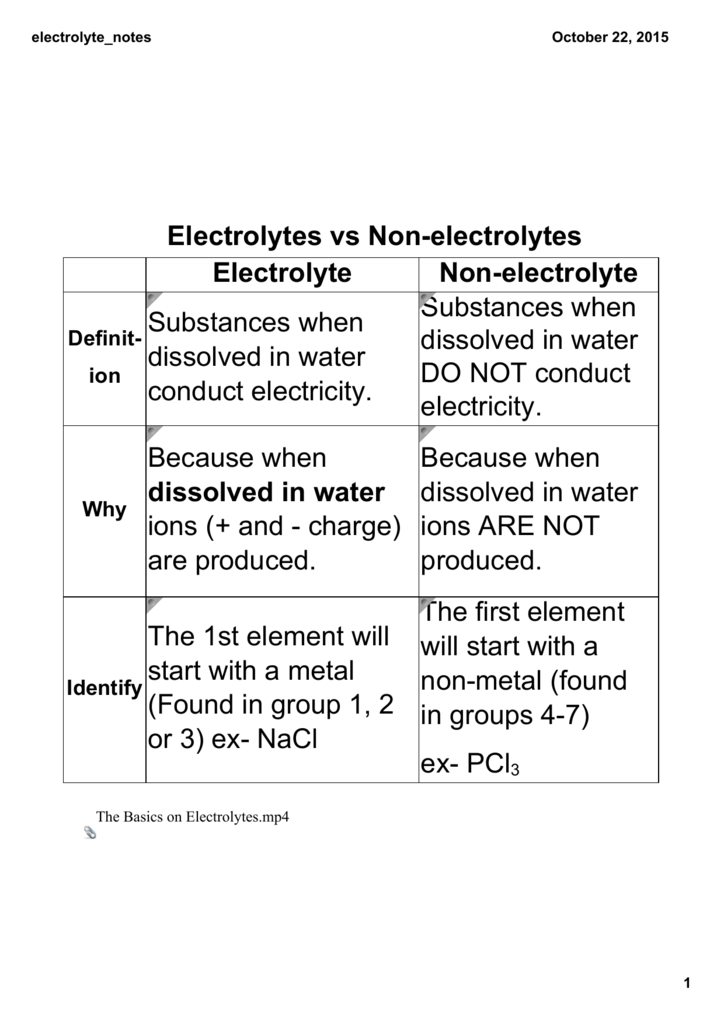 Electrolytes Vs Nonelectrolytes Electrolyte Nonelectrolyte From studylib.net
Electrolytes Vs Nonelectrolytes Electrolyte Nonelectrolyte From studylib.net
Related Post Electrolytes Vs Nonelectrolytes Electrolyte Nonelectrolyte :
The below represents a solution of ethylene glycol on the left and sodium sulfate on the right. Viewed 939 times 2 $\begingroup$ closed. A nonelectrolyte is a compound that does not conduct an electric current in either aqueous solution or in the molten state. Strong acids and strong bases are strong electrolytes [e.g., hcl(aq), h 2 so 4 (aq), hclo 4 (aq);
Some examples are oxygen, o2, ethanol, c2h5oh, and sugar, c12h22o11.
Nacl (solution) is an electrolyte in the molten. Salt, on the other hand, is a brilliant electrolyte. [closed] ask question asked 2 years, 3 months ago. One of these compounds is an electrolyte, meaning that it. This phenomenon is also the reason why solutions containing sugar do not conduct electricity. Many molecular compounds, such as sugar or ethanol , are nonelectrolytes.
 Source: numerade.com
Source: numerade.com
As a result, solutions containing nonelectrolytes will not conduct electricity. Many molecular compounds, such as sugar or ethanol , are nonelectrolytes. The mole fraction of a certain nonelectrolyte compound in a solution containing only that substance and water is 0.100.
 Source: theengineeringknowledge.com
Source: theengineeringknowledge.com
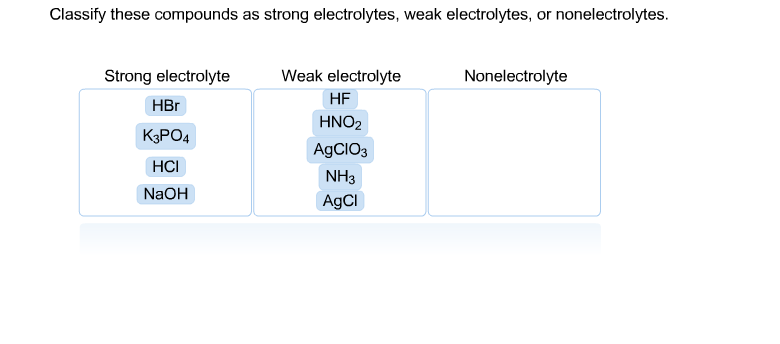
A solution containing 6g of the compound exerts the same osmotic pressure as that of 0.05 m glucose solution at the same temperature. Classify each compound as a strong electrolyte, weak electrolyte, or nonelectrolyte. Those that follow the rules of being soluble in solubility rules b.
 Source: clutchprep.com
Source: clutchprep.com
A strong electrolyte completely ionizes when dissolved in water. The molecular weight of water is 18.0 g/mol. They retain their molecular structure.
 Source: studylib.net
Source: studylib.net
The below represents a solution of ethylene glycol on the left and sodium sulfate on the right. Many molecular compounds, such as sugar or ethanol , are nonelectrolytes. Is acetic acid a nonelectrolyte?
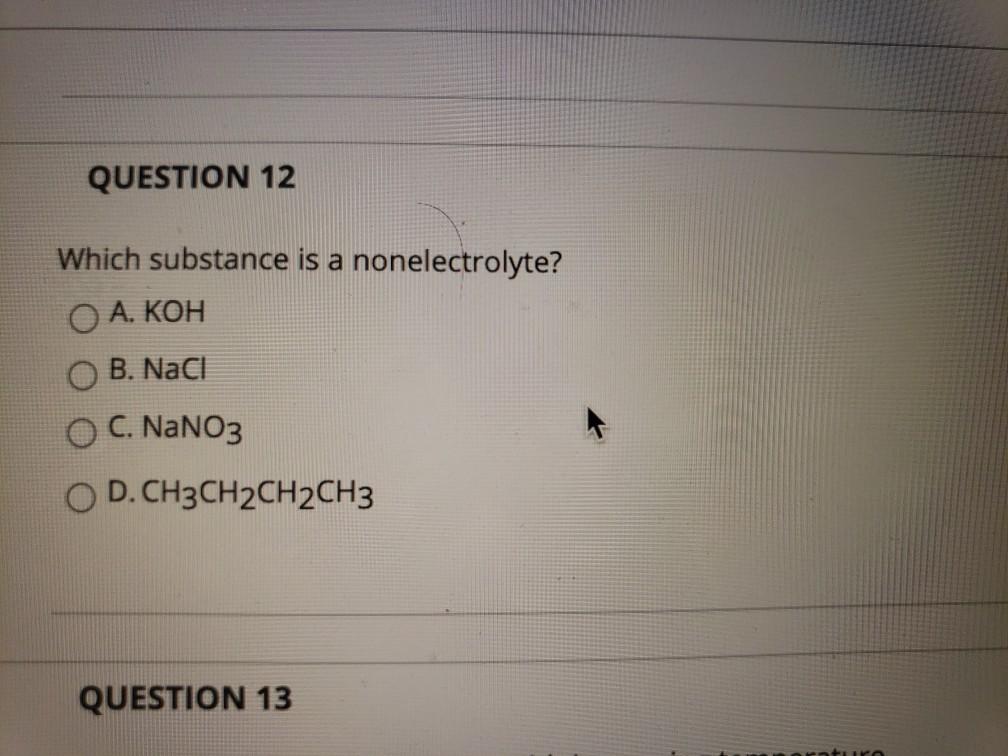
Classify each compound as a strong electrolyte, weak electrolyte, or nonelectrolyte. Nacl, (nh4)2so4, h2o2, ba (oh)2, kbr. The below represents a solution of ethylene glycol on the left and sodium sulfate on the right.
 Source: wps.prenhall.com
Source: wps.prenhall.com
Acetic acid (ch 3 cooh), the compound in vinegar, is a weak electrolyte. When these compounds dissolve in water , they do not produce ions. The below represents a solution of ethylene glycol on the left and sodium sulfate on the right.
 Source: clutchprep.com
Source: clutchprep.com
They retain their molecular structure. Classify each compound as a strong electrolyte, weak electrolyte, or nonelectrolyte. Strong electrolyte weak electrolyte nonelectrolyte.
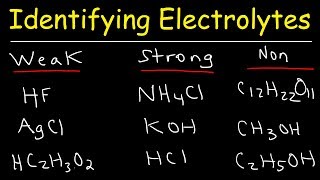 Source: youtube.com
Source: youtube.com
They share electron pairs to form such bonds. The other compound is a nonelectrolyte because it does not dissociate in water 1st attempt dsee periodic table see hint the compounds below can also be classified as electrolytes; 2.the density of the solution.
 Source: numerade.com
Source: numerade.com
This phenomenon is also the reason why solutions containing sugar do not conduct electricity. Many molecular compounds, such as sugar or ethanol, are nonelectrolytes. [closed] ask question asked 2 years, 3 months ago.
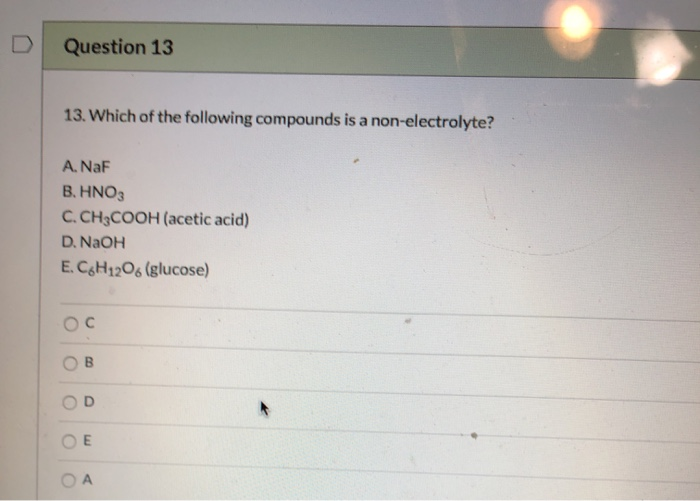 Source: chegg.com
Source: chegg.com
Viewed 939 times 2 $\begingroup$ closed. They retain their molecular structure. We’re being asked to classify each given compound as a strong electrolyte, weak electrolyte, or nonelectrolytes.recall that:
 Source: chegg.com
Source: chegg.com
- kcl (aq) 2) h2so 4 (dil) 3) ccl 4 (l) 4) ch 3 cooh (aq) class 10 icse. Hcl, hbr, hi, hno 3, h 2 so 4, hclo 4, hclo 3 Typically, nonelectrolytes are primarily held together by covalent rather than ionic bonds.
 Source: chegg.com
Source: chegg.com
Is acetic acid a nonelectrolyte? When these compounds dissolve in water , they do not produce ions. The definition of electrolyte is a substance which forms ion in an aqueous solution.
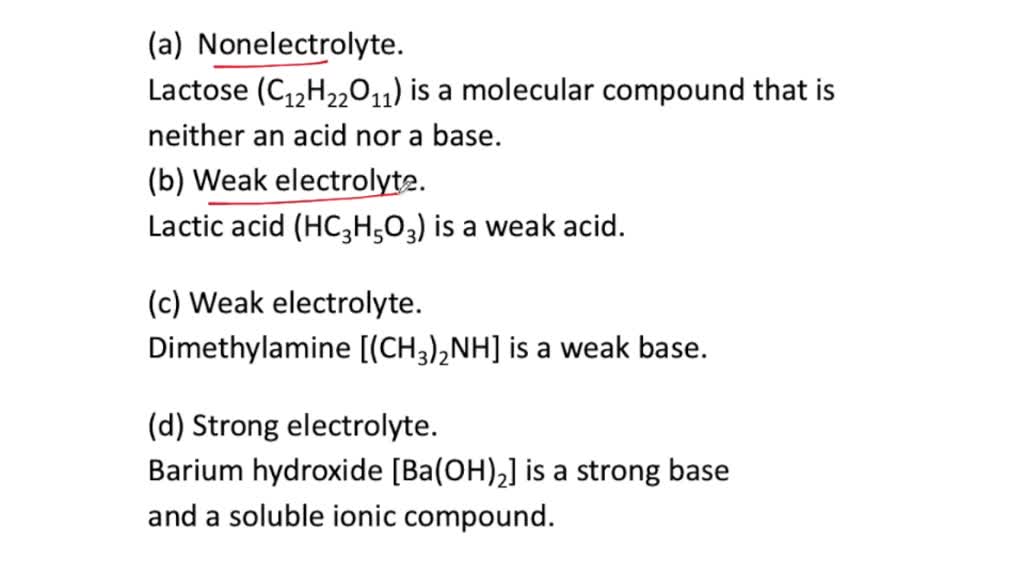
We’re being asked to classify each given compound as a strong electrolyte, weak electrolyte, or nonelectrolytes.recall that: They are compounds that do not disintegrate in polar solvents. Strong acids and strong bases are strong electrolytes [e.g., hcl(aq), h 2 so 4 (aq), hclo 4 (aq);
 Source: chegg.com
Source: chegg.com
Click again to see term 👆. The molecular formula of the compound is: Classify each compound as a strong electrolyte, weak electrolyte, or nonelectrolyte.
 Source: brainly.com
Source: brainly.com
Strong electrolyte weak electrolyte nonelectrolyte. Solutions of nonelectrolytes do not conduct electricity. Many molecular compounds, such as sugar or ethanol, are nonelectrolytes.
 Source: slideplayer.com
Source: slideplayer.com
This phenomenon is also the reason why solutions containing sugar do not conduct electricity. It does not provide ions in a solution and therefore current does not flow through such solution. All the others are electrolytes;
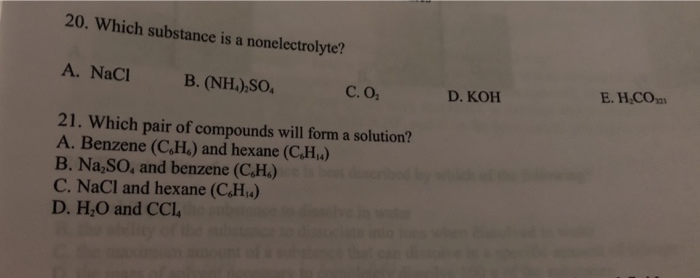 Source: chegg.com
Source: chegg.com
Tap again to see term 👆. Strong electrolyte weak electrolyte nonelectrolyte. They retain their molecular structure.
 Source: numerade.com
Source: numerade.com
As a result, solutions containing nonelectrolytes will not conduct electricity. Which substance is an electrolyte ccl4? The molecular formula of the compound is:
 Source: focuskimia.com
Source: focuskimia.com
A common example of a nonelectrolyte is glucose, or c6h12o6. We’re being asked to classify each given compound as a strong electrolyte, weak electrolyte, or nonelectrolytes.recall that: A common example of a nonelectrolyte is glucose, or c6h12o6.
 Source: kentchemistry.com
Source: kentchemistry.com
Nonelectrolytes tend to be poor electrical conductors and don�t readily dissociate into ions when melted or dissolved. Salt, on the other hand, is a brilliant electrolyte. Which substance is an electrolyte ccl4?
Also Read :





