Only aniline is a benzenoid compound as only aniline has a benzene ring which satisy the conditions of an aromatic compound, i.e. So, ch4 will have the lowest boiling point.
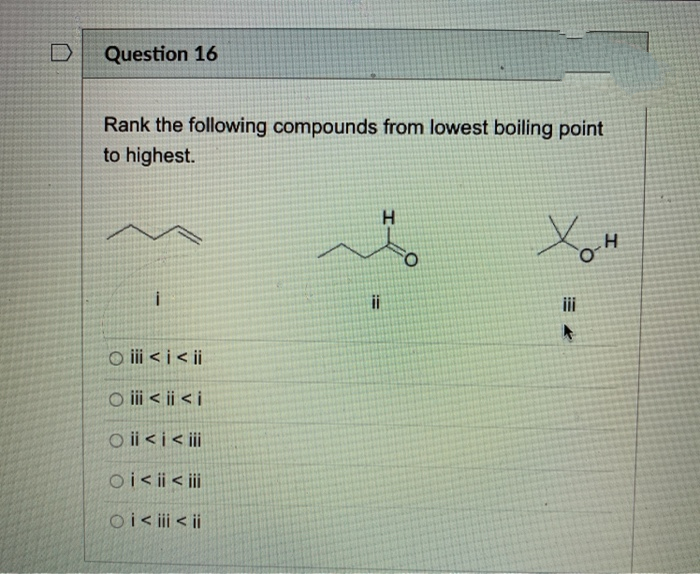
Which Compound Has The Lowest Boiling Point. Which organic compound has the lowest boiling point? Ethene has lowest boiling point. A.) only has london dispersion so has the weakest strength interactions, so lowest boiling point. The compound which has the lowest boiling point is:
 Oneclass: 6. 8 Pts. Which Compound Has The Highest Boiling Point?, Which Has The Lowest Boiling Point… From oneclass.com
Oneclass: 6. 8 Pts. Which Compound Has The Highest Boiling Point?, Which Has The Lowest Boiling Point… From oneclass.com
Related Post Oneclass: 6. 8 Pts. Which Compound Has The Highest Boiling Point?, Which Has The Lowest Boiling Point… :
Which compound has the lowest boiling point? All boiling points below are normal/atmospheric boiling points: Propane has the lowest melting and boiling points and the weakest interactions. Which structure has the lowest boiling point?
Hence the order of increasing boiling point is $$ \ce{h2s} < \ce{h2se} < \ce{h2te} < \ce{h2o} $$ and to answer your question $\ce{h2s}$ has the lowest boiling point.
So $\ce{h2te}$ has strongest dispersion force so among the rest three it has high boiling point followed by $\ce{h2se}$ and $\ce{h2s}$. A.) only has london dispersion so has the weakest strength interactions, so lowest boiling point. To convert celsius to fahrenheit or kelvin. Hence it has the lowest boiling point. So, ch4 will have the lowest boiling point. The compound which has the lowest boiling point is:
 Source: youtube.com
Source: youtube.com
Therefore diphenyl ether which has a molecular weight of 170 g/mol has much stronger london dispersion forces as compared to diethyl ether which has a molecular weight of 74 g/mol from above discussion, we can conclude that diethyl ether has the weakest intermolecular forces of attraction. Only aniline is a benzenoid compound as only aniline has a benzene ring which satisy the conditions of an aromatic compound, i.e. All boiling points below are normal/atmospheric boiling points:
 Source: chegg.com
Source: chegg.com
Hence it has the lowest boiling point. The higher boiling point of nh3 is due to excessive hydrogen bonding, so ph3 have lowest boiling point among hydrides of group number 15, i.e. All boiling points below are normal/atmospheric boiling points:
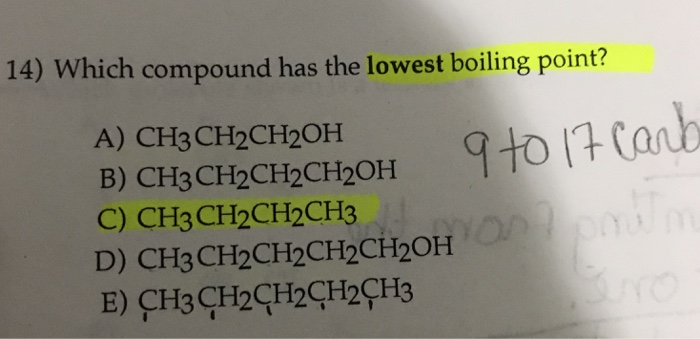
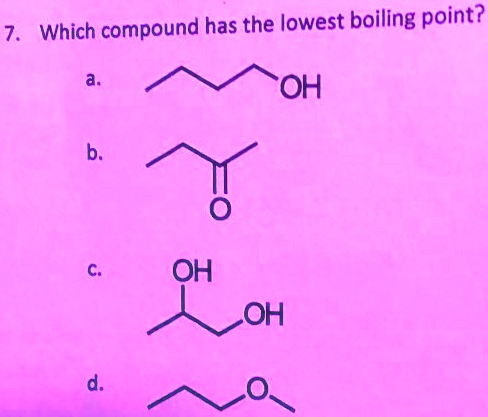
To convert celsius to fahrenheit or kelvin. Only aniline is a benzenoid compound as only aniline has a benzene ring which satisy the conditions of an aromatic compound, i.e. Therefore, the shortest, most branched molecule in this problem will have the lowest boiling point.
 Source: oneclass.com
Source: oneclass.com
Ch3ch2oh >ch3ch2f>ch3och3>ch3ch2ch2ch3>ch3ch2ch3 is the descending order of boiling point. Which of the following compound has lowest boiling point? So $\ce{h2te}$ has strongest dispersion force so among the rest three it has high boiling point followed by $\ce{h2se}$ and $\ce{h2s}$.
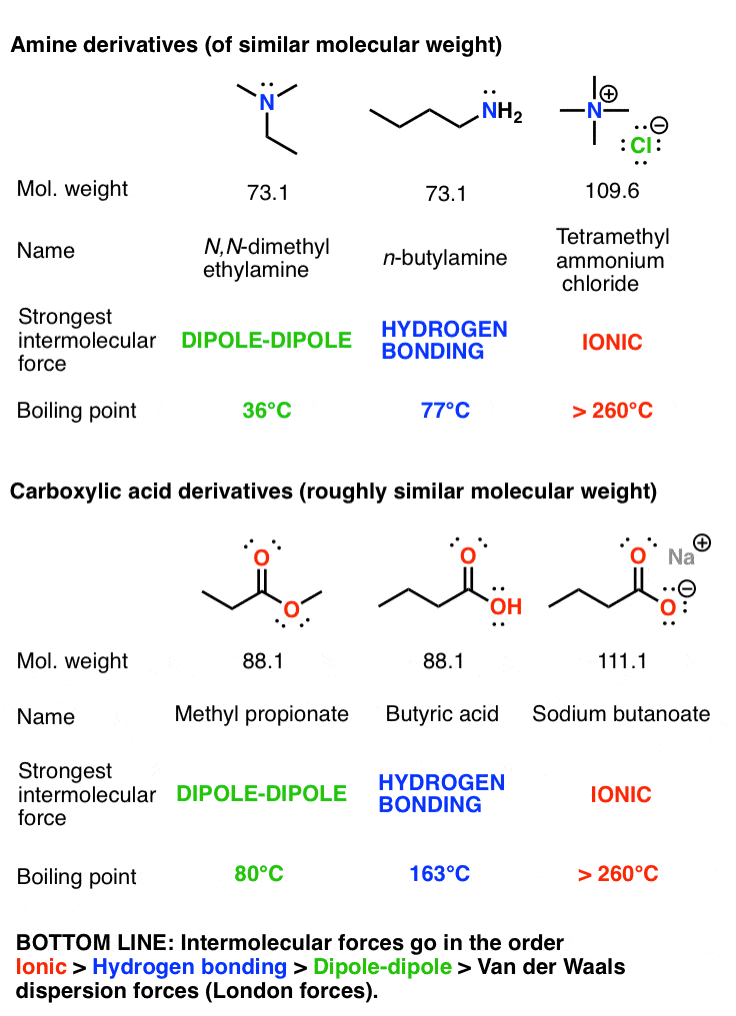 Source: masterorganicchemistry.com
Source: masterorganicchemistry.com
>> alcohols, phenols and ethers. Has planar structure (ring) ii. Point (o c) boiling point (o c) state at 25 o c:
 Source: chemistry.stackexchange.com
Source: chemistry.stackexchange.com
To convert celsius to fahrenheit or kelvin. The chemical element with the lowest boiling point is helium and the element with the highest boiling point is tungsten. The higher boiling point of nh3 is due to excessive hydrogen bonding, so ph3 have lowest boiling point among hydrides of group number 15, i.e.
 Source: study.com
Source: study.com
Ch3ch2oh >ch3ch2f>ch3och3>ch3ch2ch2ch3>ch3ch2ch3 is the descending order of boiling point. Which compound has the lowest boiling point? Ch4, has the lowest boiling point.
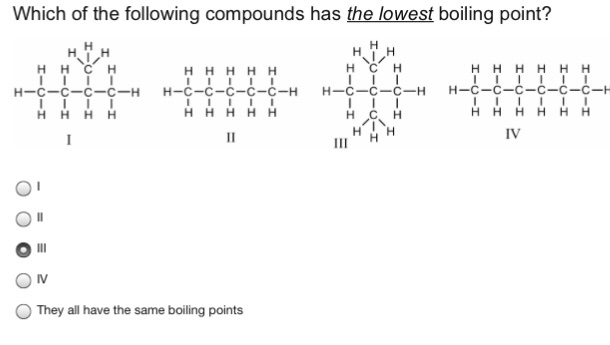
Which compound has the lowest boiling point? It has been found that helium has the lowest normal boiling point (−268.9 °c) because it has very weak intermolecular attractions. Ch3ch2oh >ch3ch2f>ch3och3>ch3ch2ch2ch3>ch3ch2ch3 is the descending order of boiling point.

It has been found that helium has the lowest normal boiling point (−268.9 °c) because it has very weak intermolecular attractions. Ch3ch2oh >ch3ch2f>ch3och3>ch3ch2ch2ch3>ch3ch2ch3 is the descending order of boiling point. The chemical element with the lowest boiling point is helium and the element with the highest boiling point is tungsten.
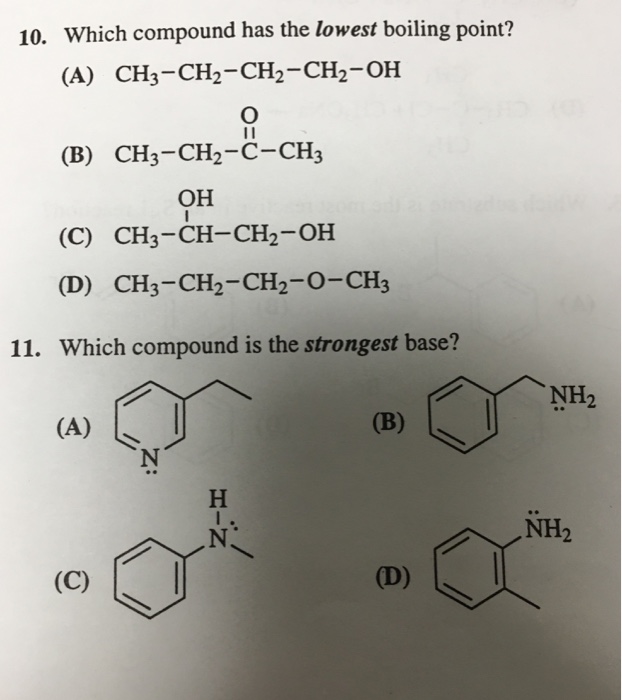
So, ch4 will have the lowest boiling point. Which compound has lowest boiling point? The chemical element with the lowest boiling point is helium and the element with the highest boiling point is tungsten.
![Solved] Which Compound Has The Lowest Boiling Point? | Course Hero](https://www.coursehero.com/qa/attachment/16046178/ “Solved] Which Compound Has The Lowest Boiling Point? | Course Hero”) Source: coursehero.com
Which compound has the highest boiling point? The correct answer is isobutane, a four membered, branched hydrocarbon. It has been found that helium has the lowest normal boiling point (−268.9 °c) because it has very weak intermolecular attractions.
 Source: studylib.net
Source: studylib.net
The one with the weakest imf will have the lowest boiling point. Hence the order of increasing boiling point is $$ \ce{h2s} < \ce{h2se} < \ce{h2te} < \ce{h2o} $$ and to answer your question $\ce{h2s}$ has the lowest boiling point. Helium has the lowest boiling point of all the element.because of only weak van dar waal force between them… van der waal force increases with weight.and that�s why helium is.
 Source: youtube.com
Source: youtube.com
Boiling points of alkanes increase as the number of carbon atom increase or molecular mass increase. So $\ce{h2te}$ has strongest dispersion force so among the rest three it has high boiling point followed by $\ce{h2se}$ and $\ce{h2s}$. The unity used for the melting point is celsius (c).
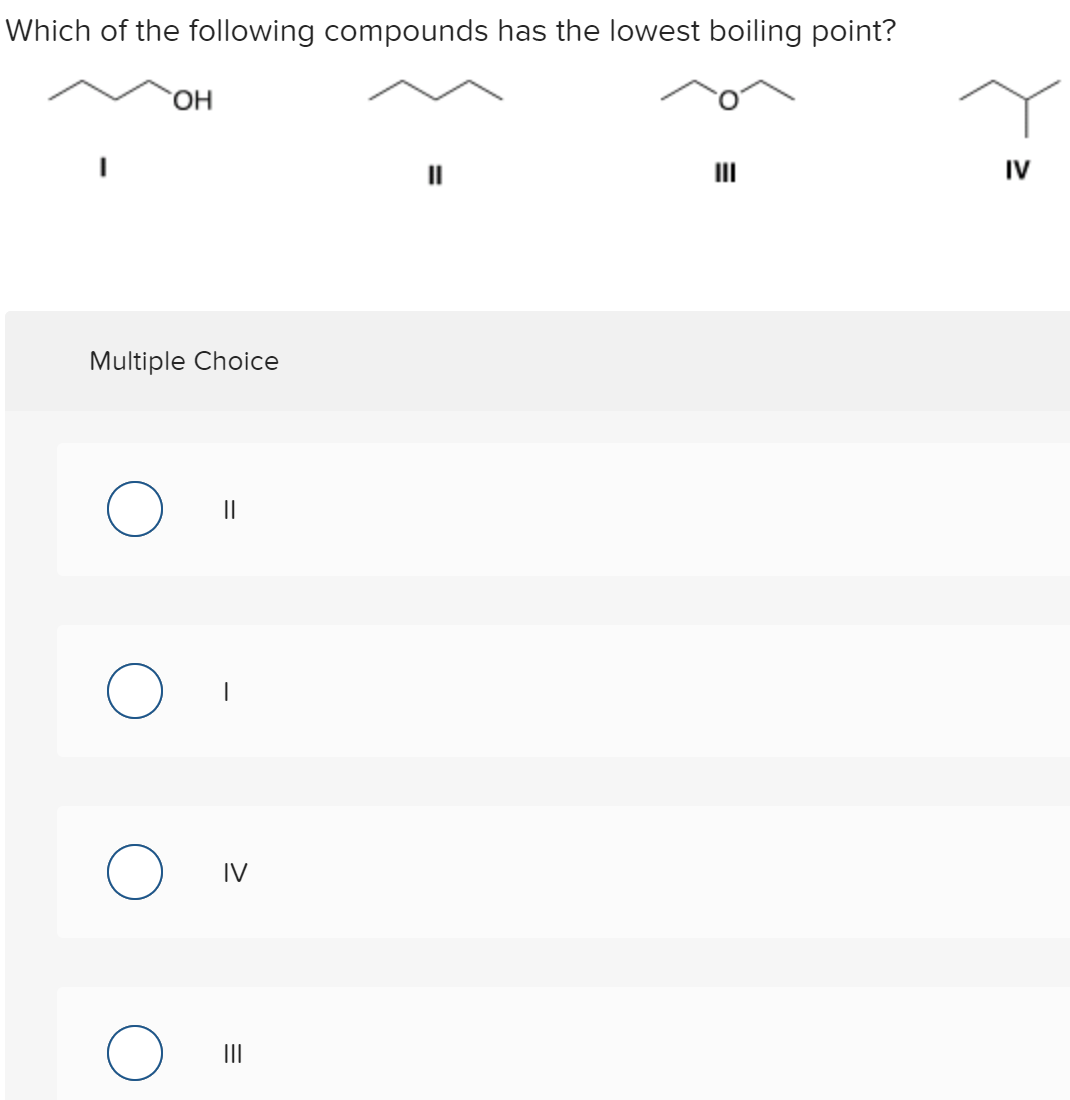 Source: bartleby.com
Source: bartleby.com
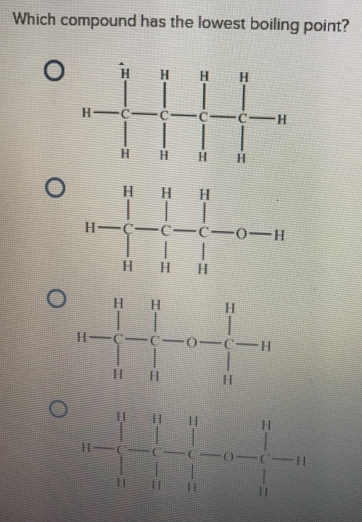
Boiling points of alkanes increase as the number of carbon atom increase or molecular mass increase. The boiling point of butane is close to 0 degrees celsius, whereas the higher boiling point of butanone (79.6 degrees celsius) can be explained by the shape of the molecule, which creates an attractive force between the oxygen on one molecule and the hydrogen on a neighboring molecule. Ionic compounds are formed from strong electrostatic interactions between ions, which result in higher melting points and electrical conductivity compared to covalent compounds.
 Source: chemistrysteps.com
Source: chemistrysteps.com
Which structure has the lowest boiling point? Nitrogen (n2), carbon dioxide, oxygen (o2), helium, chlorine (cl2) and hydrogen are all familiar examples of substances that boil at much lower temperatures than water. A.) only has london dispersion so has the weakest strength interactions, so lowest boiling point.
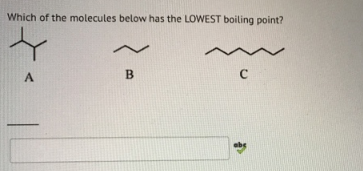 Source: clutchprep.com
Source: clutchprep.com
Now since the other halogens can not show hydrogen bonding…their boiling point is determined on the basis of their molecular weight.it is because for hcl hbr hi the boiling point is determined by the van der waals force of attraction which depends on the surface area of the molecule.now in between hcl hbr and hi the surface area of hi is the largest and hcl has the. The one with the weakest imf will have the lowest boiling point. The boiling point of a liquid at 1 atm is also known as the normal boiling point.
 Source: bartleby.com
Source: bartleby.com
>> alcohols, phenols and ethers. To convert celsius to fahrenheit or kelvin. Which compound has the highest boiling point?
 Source: clutchprep.com
Source: clutchprep.com
The boiling point of butane is close to 0 degrees celsius, whereas the higher boiling point of butanone (79.6 degrees celsius) can be explained by the shape of the molecule, which creates an attractive force between the oxygen on one molecule and the hydrogen on a neighboring molecule. Which compound has lowest boiling point? Point (o c) boiling point (o c) state at 25 o c:
 Source: slideplayer.com
Source: slideplayer.com
To convert celsius to fahrenheit or kelvin. Ch4, has the lowest boiling point. Therefore diphenyl ether which has a molecular weight of 170 g/mol has much stronger london dispersion forces as compared to diethyl ether which has a molecular weight of 74 g/mol from above discussion, we can conclude that diethyl ether has the weakest intermolecular forces of attraction.
![Solved:which Compound Has The Lowest Boiling Point?] 7. Oh Oh Oh](https://cdn.numerade.com/ask_images/8b464be79f3e4382bc62da415eb206eb.jpg “Solved:which Compound Has The Lowest Boiling Point?] 7. Oh Oh Oh”) Source: numerade.com
Propane has the lowest melting and boiling points and the weakest interactions. The boiling point of a liquid at 1 atm is also known as the normal boiling point. Hence the order of increasing boiling point is $$ \ce{h2s} < \ce{h2se} < \ce{h2te} < \ce{h2o} $$ and to answer your question $\ce{h2s}$ has the lowest boiling point.
Also Read :