Cas na n 3 mgso correct! Oxidation number of carbon in hcho= 12×1−2.
Which Compound Has The Atom With The Highest Oxidation Number. For instance, na + ion has the oxidation number +1. Atoms chemical kinetics moving charges and magnetism microbes in human welfare semiconductor electronics: All alkali metals in the compound form will have oxidation number +1. 1.) â which compound has the atom wih the highest oxidation number.
 Solved Which Compound Has The Atom With The Highest | Chegg.com From chegg.com
Solved Which Compound Has The Atom With The Highest | Chegg.com From chegg.com
Related Post Solved Which Compound Has The Atom With The Highest | Chegg.com :
Which compound has the atom with the highest oxidation number? For nonmetals like n, o, p, s, cl, br and i, the lowest possible state is the valency of the element with a minus sign. Each atom in an element either in its free or uncombined state holds up an oxidation number of zero. Which compound has the atom with the highest oxidation number?
Click to see full answer.
Mgso4 what volume of a concentrated solution of sodium hydroxide 6.00must be diluted to 200.0ml to make a 0.880 m solution of sodium hydroxide? In respect to this, which compound has the atom with the highest oxidation number? Manganese has the highest oxidation state because the number of unpaired electrons in the outermost shell is more, i.e. Which compound has the atom with the highest oxidation number?a. Which elements have the +7 oxidation number and give examples? 1.) â which compound has the atom wih the highest oxidation number.
 Source: youtube.com
Source: youtube.com
Cas na n 3 mgso correct! Which compound has the atom with the highest oxidation number?a. The metals in group ia form compounds (such as li 3 n and na 2 s) in which the metal atom has an oxidation number of +1.
 Source: youtube.com
Source: youtube.com
Yes you are right sulphur has the highest oxidation number. View solution > the pair of compounds having metals in their highest oxidation state is: Ions having one atom bear oxidation number equal to the charge present on the ion.

This rule will apply to all ions. Which compound has the atom with the highest oxidation number?a. Manganese, which is in the middle of the period, has the highest number of oxidation states, and indeed the highest oxidation state in the whole period.

Which compound has the atom with the highest oxidation number? Hcooh has highest oxidation state. For instance, na + ion has the oxidation number +1.
 Source: doubtnut.com
Source: doubtnut.com
Alkali metals (group 1 metals) like. Mgso4 what volume of a concentrated solution of sodium hydroxide 6.00must be diluted to 200.0ml to make a 0.880 m solution of sodium hydroxide? Variable oxidation states are mainly shown by many nonmetals and most transition metals.
 Source: chegg.com
Source: chegg.com
93% (15 ratings) transcribed image text: Mgso4 what volume of a concentrated solution of sodium hydroxide 6.00must be diluted to 200.0ml to make a 0.880 m solution of sodium hydroxide? Which elements have the +7 oxidation number and give examples?
 Source: numerade.com
Source: numerade.com
Group vii elements form highest oxidation numbers. Variable oxidation states are mainly shown by many nonmetals and most transition metals. Ions having one atom bear oxidation number equal to the charge present on the ion.

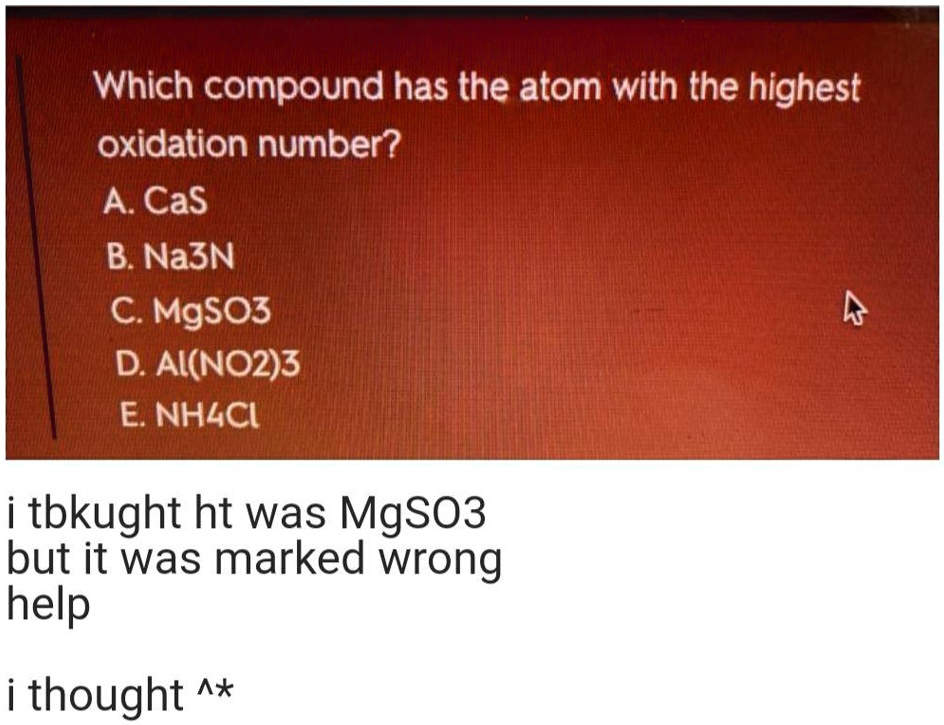
The most common species of nitrogen in which its oxidation state is zero is the diatomic nitrogen ( ). 29) which compound has the atom with the highest oxidation number a) na3n b) ai (no2)3 c) nh4c d) cas e) mgso3. Its lowest oxidation state is zero in mn2 (co)10.
 Source: chegg.com
Source: chegg.com
Oxidation number of carbon in hcooh= 12×2−2. Which compound has the atom with the highest oxidation number? In p block elements, what is the maximum oxidation number?
 Source: chegg.com
Source: chegg.com
Casâ na3n mgso3â al(no2)3 nh4cl 2.)â what name (label) is given to the hybridized orbitals of sf6. Ions having one atom bear oxidation number equal to the charge present on the ion. This rule will apply to all ions.
 Source: chegg.com
Source: chegg.com
Manganese has the highest oxidation state because the number of unpaired electrons in the outermost shell is more, i.e. Which elements have the +7 oxidation number and give examples? Manganese has the highest oxidation state because the number of unpaired electrons in the outermost shell is more, i.e.
 Source: chegg.com
Source: chegg.com
The oxidation number of ions which comprise of only one atom is equal to the actual charge on the ion. Compound in which the oxidation number of nitrogen is +1 is : The oxidation number of each atom can be calculated by subtracting the sum of lone pairs and electrons it gains from bonds from the number of valence electrons.
 Source: clutchprep.com
Source: clutchprep.com
Alkali metals (group 1 metals) like. Correct option is d) among the given compound, i shows the highest oxidation state and it is + 7. Mgso4 what volume of a concentrated solution of sodium hydroxide 6.00must be diluted to 200.0ml to make a 0.880 m solution of sodium hydroxide?
 Source: numerade.com
Source: numerade.com
Atoms chemical kinetics moving charges and magnetism microbes in human welfare semiconductor electronics: Yes you are right sulphur has the highest oxidation number. Sulfur has highest oxidation number for.
 Source: chegg.com
Source: chegg.com
For instance, na + ion has the oxidation number +1. The most common species of nitrogen in which its oxidation state is zero is the diatomic nitrogen ( ). Which elements have the +7 oxidation number and give examples?
 Source: clutchprep.com
Source: clutchprep.com
Group vii elements form highest oxidation numbers. The oxidation number of ions which comprise of only one atom is equal to the actual charge on the ion. Manganese has the highest oxidation state because the number of unpaired electrons in the outermost shell is more, i.e.
 Source: chegg.com
Source: chegg.com
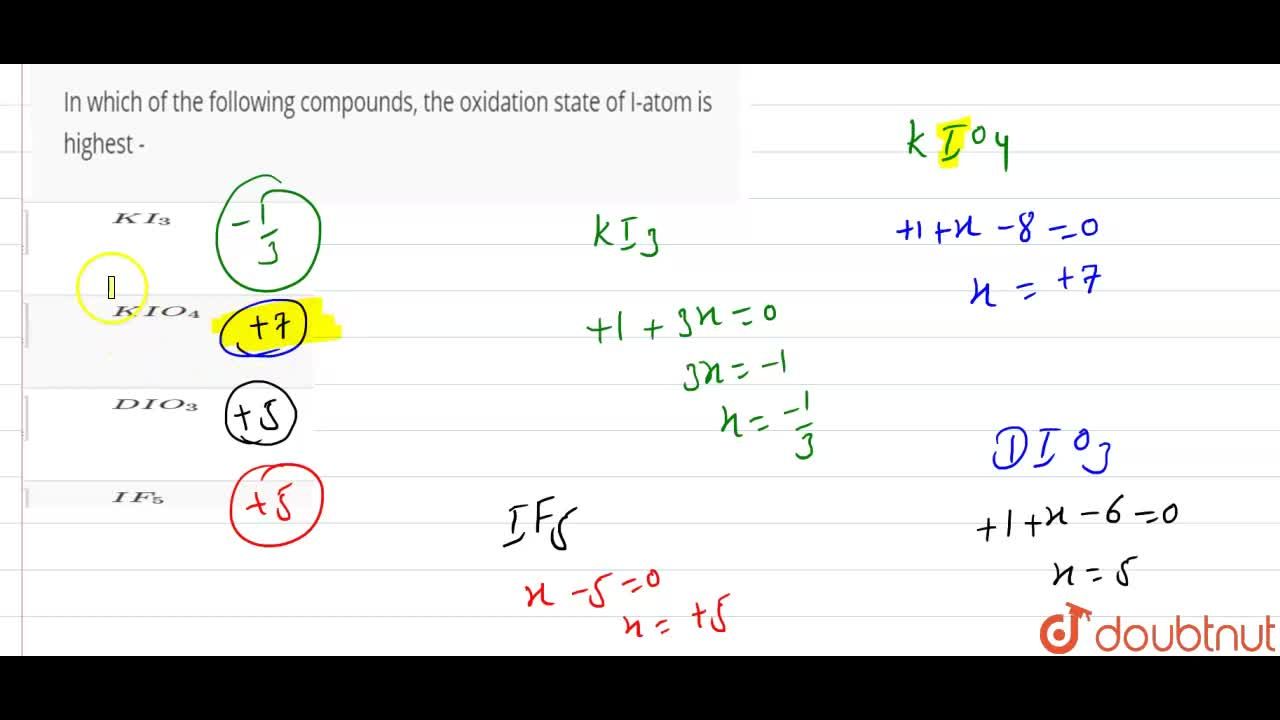
Which compound has the atom with the highest oxidation number?a. K i o 4 medium. By checking which atom is bonded to elements more electronegative than it is, and generally the atom with the highest oxidation number takes a central position in the molecule.
 Source: numerade.com
Source: numerade.com
Experts are tested by chegg as specialists in their subject area. For instance, na + ion has the oxidation number +1. Explain how this label applies to the orbitals of the free atoms.
 Source: chegg.com
Source: chegg.com
Experts are tested by chegg as specialists in their subject area. Atoms chemical kinetics moving charges and magnetism microbes in human welfare. Cas na n 3 mgso correct!

Electronegativity is basically how strongly that element draws electrons to itself. Which compound has the atom with the highest oxidation number? Compound in which the oxidation number of nitrogen is +1 is :
Also Read :