When both ionic and covalent bonding occurs in a compound, the ionic portion is almost always between the cation and anion of the compound. | snapsolve the compound which contains both ionic ad covalent bonds is ?
Which Compound Has Both Ionic And Covalent Bonds. These species share an ionic bond, while the carbon and oxygen atoms in carbonate are covalently bonded. Comparison of ionic and covalent bonds ionic bonds usually occur between. For example, sodium (na), a metal, and chloride (cl), a nonmetal, form an ionic bond to make nacl. Naphthalene, as a covalent compound, is made up of covalent molecules only.
 Solved 1.32 Each Compound Contains Both Ionic And Covalent | Chegg.com From chegg.com
Solved 1.32 Each Compound Contains Both Ionic And Covalent | Chegg.com From chegg.com
Related Post Solved 1.32 Each Compound Contains Both Ionic And Covalent | Chegg.com :
Which compound contains both ionic and covalent bonds? These species share an ionic bond, whereas the carboncarbon Why is it true to say that calcium carbonate has both ionic and covalent bonds? | snapsolve the compound which contains both ionic ad covalent bonds is ?
Caco3 has both ionic and covalent bonds, ionic between ca and co3 and covalent within co3.
When naoh is dissolved in water, the equation follows: In a covalent bond, the atoms bond by sharing electrons. (a) caco3 (b)ch2cl2 (c)ch3oh (d)c6h12o6 A proton (h+) could form a coordinate covalent bond with a. (1) caco3 is the correct answer. Here calcium acts as the cation, with the carbonate species as the anion.
 Source: toppr.com
Source: toppr.com
Which kinds of bonds are found in a sample of h2o(s)? Calcium carbonate is another example of a compound with both ionic and covalent bonds. Caf2 is an ionic compound because, in the caf2 molecule, calcium acts as a cation by donating its 2 extra electrons from the valence shell, and fluoride acts as an anion by accepting the electrons from calcium, completing its octet.
 Source: slidetodoc.com
Source: slidetodoc.com
(1) caco3 is the correct answer. Which compound has both ionic and covalent bonding? The covalent bonds could occur in a polyatomic ion in either the cation or the anion.
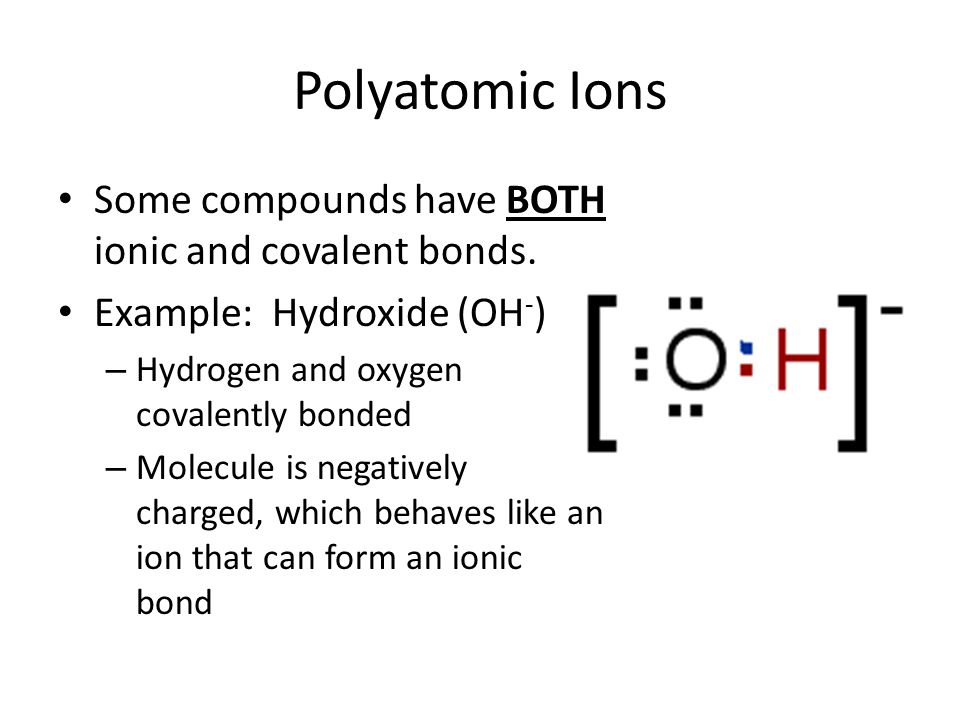 Source: slideplayer.com
Source: slideplayer.com
Comparison of ionic and covalent bonds ionic bonds usually occur between. This 13 words question was answered by heather l. A) n h 4 c l contains both an iconic and covalent bond.
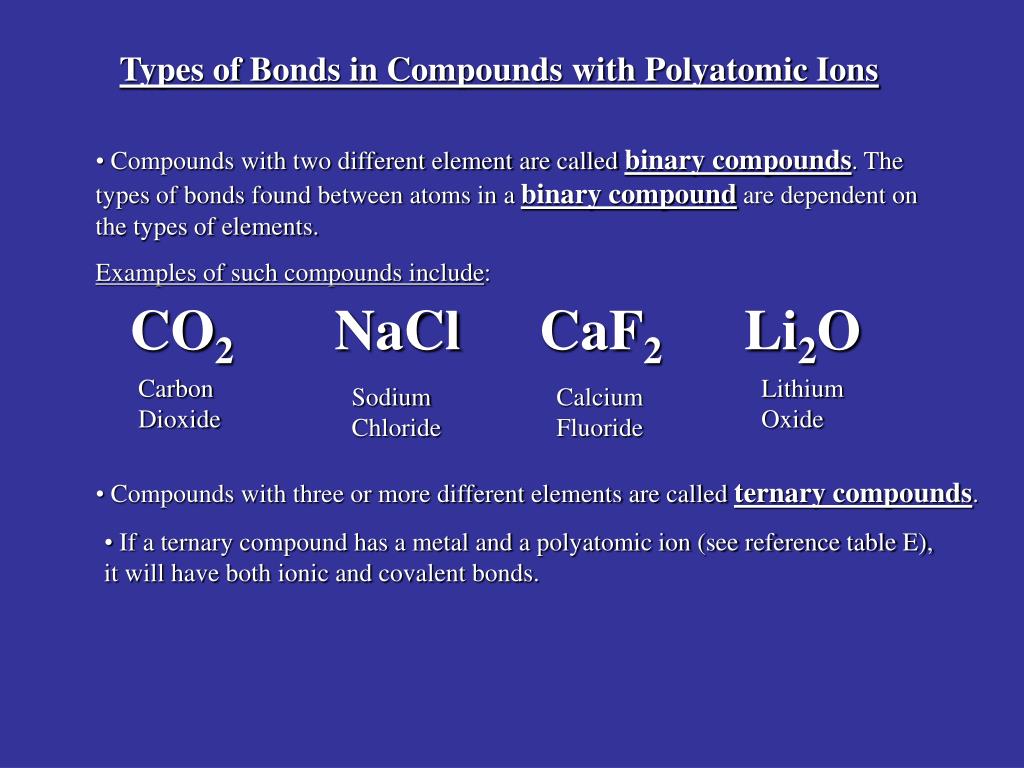 Source: slideserve.com
Source: slideserve.com
Caco3 has both ionic and covalent bonds, ionic between ca and co3 and covalent within co3. For example, sodium (na), a metal, and chloride (cl), a nonmetal, form an ionic bond to make nacl. Here calcium acts as the cation, with the carbonate species as the anion.
 Source: slideplayer.com
Source: slideplayer.com
Therefore whatever bonds it makes are ionic. The ammonium ion is polyatomic, which means it forms ionic salts. We can distinguish between them a priori by asking ourselves whether there is a contiguous chain of mostly covalent bonds between each side of the ionic interaction.
 Source: clutchprep.com
Source: clutchprep.com
(1) caco3 is the correct answer. The compound that has both ionic and covalent bonding is. The ammonium ion is polyatomic, which means it forms ionic salts.
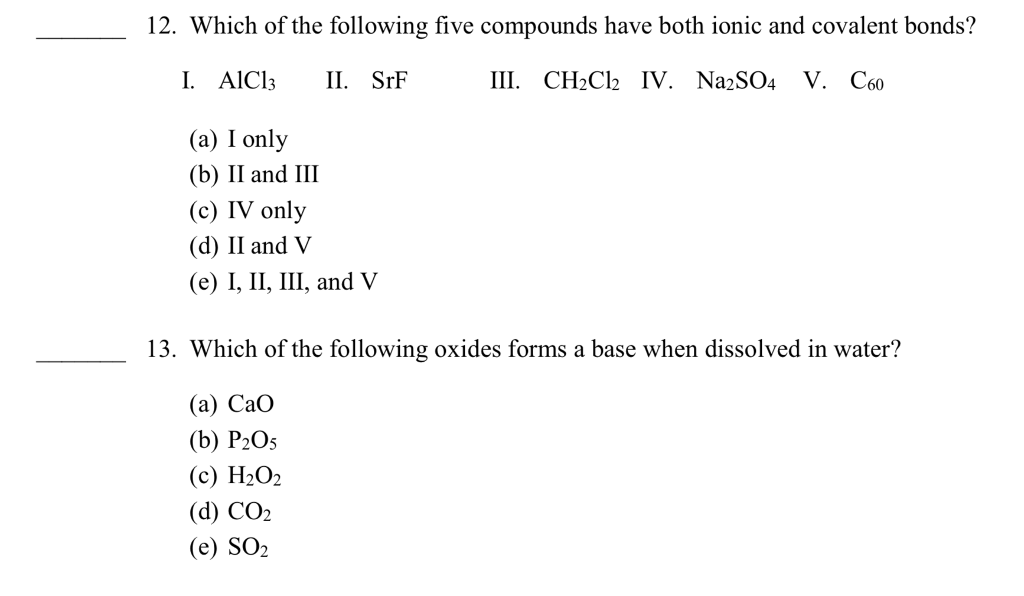 Source: chegg.com
Source: chegg.com
In other words, each ion contains more than one atoms. When both ionic and covalent bonding occurs in a compound, the ionic portion is almost always between the cation and anion of the compound. In kcn, the bonding between potassium ion and cyanide ion is ionic while carbon and nitrogen are covalently bonded in cyanide ion as:

These species share an ionic bond, while the carbon and oxygen atoms in carbonate are covalently bonded. Comparison of ionic and covalent bonds ionic bonds usually occur between. A proton (h+) could form a coordinate covalent bond with a.
 Source: toppr.com
Source: toppr.com
Calcium carbonate is one other instance of a compound with each ionic and covalent bonds. These species share an ionic bond, while the carbon and oxygen atoms in carbonate are covalently bonded. In other words, each ion contains more than one atoms.
 Source: chegg.com
Source: chegg.com
A)the entropy of the atoms b)the atomic number of the atoms c)the first ionization energy of the atoms d)the polarity of the bond. In other words, each ion contains more than one atoms. Naphthalene, as a covalent compound, is made up of covalent molecules only.
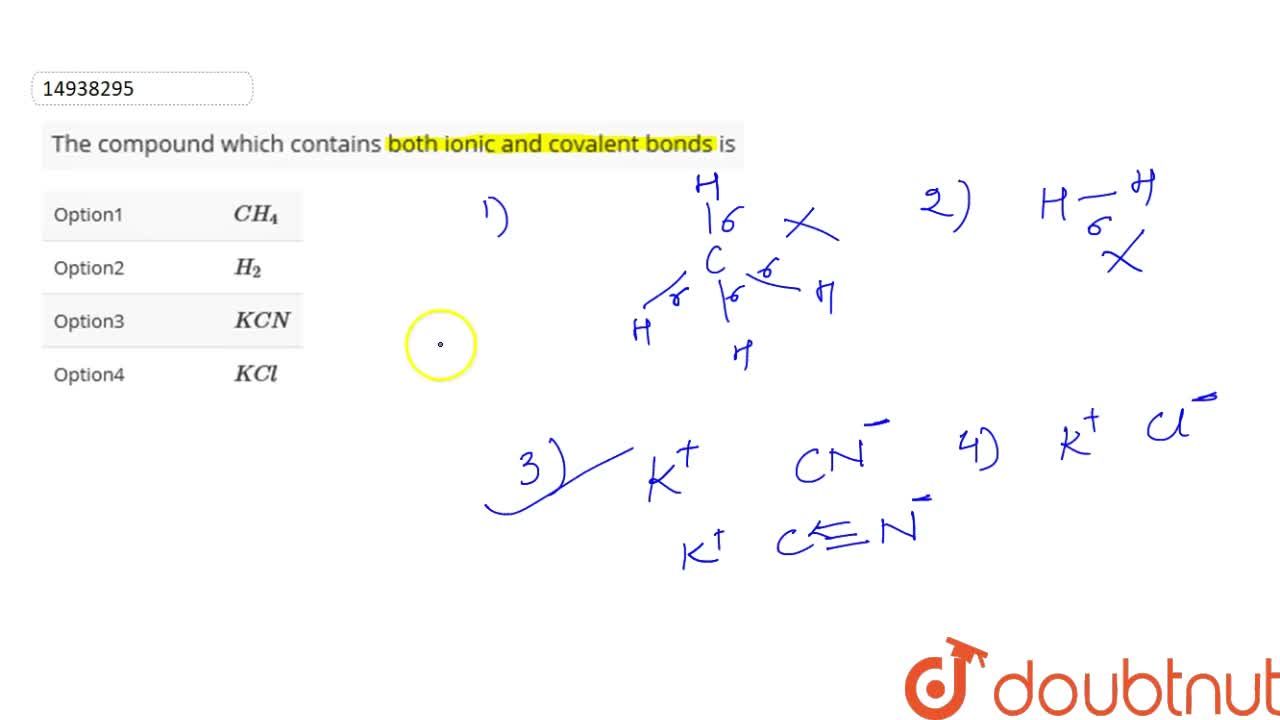 Source: doubtnut.com
Source: doubtnut.com
A proton (h+) could form a coordinate covalent bond with a. A molecule or compound is made when two or more atoms form a chemical bond that links them together. We can distinguish between them a priori by asking ourselves whether there is a contiguous chain of mostly covalent bonds between each side of the ionic interaction.
 Source: clutchprep.com
Source: clutchprep.com
Usually, there is some polarity (polar covalent bond) in which the electrons are shared, but spend more time with one atom than the other. The ammonium ion is polyatomic, which means it forms ionic salts. A molecule or compound is made when two or more atoms form a chemical bond that links them together.
 Source: brainly.in
Source: brainly.in
A)the entropy of the atoms b)the atomic number of the atoms c)the first ionization energy of the atoms d)the polarity of the bond. Therefore, has both ionic and covalent bonds. Here calcium acts as the cation, with the carbonate species as the anion.
 Source: chegg.com
Source: chegg.com
Covalent bonds usually occur between nonmetals. Which kinds of bonds are found in a sample of h2o(s)? Both covalent and hydrogen bonds 29.
 Source: slideserve.com
Source: slideserve.com
The covalent bonds could occur in a polyatomic ion in either the cation or the anion. The compound that has both ionic and covalent bonding is. (1) caco3 is the correct answer.

| snapsolve the compound which contains both ionic ad covalent bonds is ? The ammonium ion is polyatomic, which means it forms ionic salts. The and ions in are connected with ionic bonds.
 Source: intelwriters.com
Source: intelwriters.com
These atoms (one atom and four atoms) are connected with covalent bonds. The compound that has both ionic and covalent bonding is. Therefore whatever bonds it makes are ionic.
 Source: sciencenotes.org
Source: sciencenotes.org
These species share an ionic bond, whereas the carboncarbon When naoh is dissolved in water, the equation follows: A)the entropy of the atoms b)the atomic number of the atoms c)the first ionization energy of the atoms d)the polarity of the bond.
 Source: thoughtco.com
Source: thoughtco.com
Hence, an aqueous solution of magnesium chloride can conduct electricity. If there is no link between the two ionic fragments, we should call the compound an ionic one (usually ‘salt’). When naoh is dissolved in water, the equation follows:
 Source: clutchprep.com
Source: clutchprep.com
In other words, each ion contains more than one atoms. The ammonium ion is polyatomic, which means it forms ionic salts. (1) caco3 is the correct answer.
Also Read :





