They're brittle and have metallic luster d. They are generally poor conductors of heat and electricity.
Which Characteristics Describe Most Nonmetals In The Solid Phase. Nonmetals display a wide range of chemical properties and reactivities. 2) they are malleable and lack metallic luster. Solid nonmetals are generally brittle, with little or no metallic luster. Which characteristics describe most nonmetals in the solid phase.
 Nonmetal - Wikipedia From en.wikipedia.org
Nonmetal - Wikipedia From en.wikipedia.org
Related Post Nonmetal - Wikipedia :
0 g changed from 50. Nonmetals have high ionization energies and electronegativities. They�re brittle and lack metallic luster Which characteristics describe most nonmetals in the solid phase they are brittle and lack metallic luster which element has the highest electrical conductivity
Shells) in which they are placed.
Do metalloids have characteristics of both metals and nonmetals? Metals are generally known to be good conductors of heat and electricity as opposed to non metals that are known to be poor at both. Which characteristics describe most nonmetals in the solid phase? 2 🔴 on a question which characteristics describe most nonmetals in the solid phase. Most nonmetals have the properties of f) high ionization energy and poor electrical conductivity. Which characteristics describe most nonmetals in the solid phase.
 Source: brainly.com
Source: brainly.com
Most nonmetals have the ability to gain electrons easily. (4)they are brittle and lack metallic luster. They�re brittle and have metallic luster d.
 Source: mdpi.com
Source: mdpi.com
Which characteristics describe most nonmetals in the solid phase? Previous post previous which characteristics describe most nonmetals in the solid phase. Metals are generally known to be good conductors of heat and electricity as opposed to non metals that are known to be poor at both.
 Source: en.wikipedia.org
Source: en.wikipedia.org
Which characteristics describe most nonmetals in the solid phase? A sample of an element is malleable and can Leave a reply cancel reply.
 Source: en.wikipedia.org
Source: en.wikipedia.org
In liquid or gas, the particles can move a lot more than in a solid metal. Nonmetals have high ionization energies and electronegativities. Is this a good thing or a bad thing, explain your answer.
 Source: quizlet.com
Source: quizlet.com
Is this a good thing or a bad thing, explain your answer. They�re brittle and have metallic luster d. (1) good conductors of electricity (2) good conductors of heat (3) malleable (4) brittle.
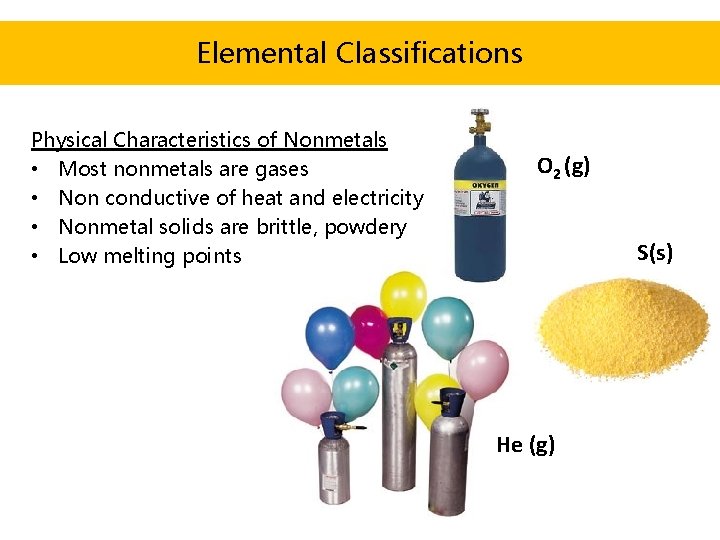 Source: slidetodoc.com
Source: slidetodoc.com
Most nonmetals have the ability to gain electrons easily. As the atomic number of elements within group 2 increases, the metallic character of each successive element 1. The physical characteristics of a non metal are not ductilemeaning it cant be drawn into wires non mallable meaning it cant berolled into thin sheets.
Metals are usually solid at room temperature. 3) they are brittle and have metallic luster. 0 g changed from 50.
 Source: thoughtco.com
Source: thoughtco.com
Nonmetals have high ionization energies and electronegativities. A sample of an element is malleable and can Compared to a neon atom, a helium atom has a 1) smaller radius 2) smaller first ionization energy
 Source: slideplayer.com
Source: slideplayer.com
Your email address will not be published. Nonmetals display a wide range of chemical properties and reactivities. Which characteristics describe most nonmetals in the solid phase.
 Source: angelo.edu
Source: angelo.edu
The atoms interact in an effort to complete themselves (fill valence. Nonmetals display a wide range of chemical. They retain the same mass and shape regardless of outside influences.
 Source: study.com
Source: study.com
Do metalloids have characteristics of both metals and nonmetals? Solid nonmetals are generally brittle, with little or no metallic luster. Nonmetals display a wide range of.

Your email address will not be published. In chemistry, a nonmetal is a chemical element that usually gains electrons when reacting with a metal, and which forms an acid if combined with oxygen and hydrogen, or which is a poor conductor of heat and electricity. To recap, the main character traits of a solid are as follows:
 Source: yumpu.com
Source: yumpu.com
Which characteristics describe most nonmetals in the solid phase. Leave a reply cancel reply. Solid nonmetals are generally brittle, with little or no metallic luster.
 Source: sciencedirect.com
Source: sciencedirect.com
A crystalline solid has a high melting point and is a good conductor of electricity in the liquid state. Your email address will not be published. Most nonmetals have the ability to gain electrons easily.
 Source: coursehero.com
Source: coursehero.com
Metals are usually solid at room temperature. Nonmetals display more variety in color and state than do metals. Shells) in which they are placed.
 Source: researchgate.net
Source: researchgate.net
Which characteristics describe most nonmetals in the solid phase? Most nonmetals have the ability to gain electrons easily. 2) they are malleable and lack metallic luster.
 Source: slideplayer.com
Source: slideplayer.com
Which characteristics describe most nonmetals in the solid phase? Shells) in which they are placed. The individual atoms coalesce to define the properties of the objects.
 Source: yumpu.com
Source: yumpu.com
Required fields are marked * comment. Which characteristic describes most nonmetals the solid phase? Leave a reply cancel reply.
 Source: en.wikipedia.org
Source: en.wikipedia.org
Which characteristics describe most nonmetals in the solid phase they are brittle and lack metallic luster which element has the highest electrical conductivity Most nonmetals have the ability to gain electrons easily. Next post next the temperature of a sample of iron with a mass of 10.
 Source: howtodiscuss.com
Source: howtodiscuss.com
A crystalline solid has a high melting point and is a good conductor of electricity in the liquid state. Why are most (around 90%) of elements in their solid phase. A sample of an element is malleable and can
Also Read :





