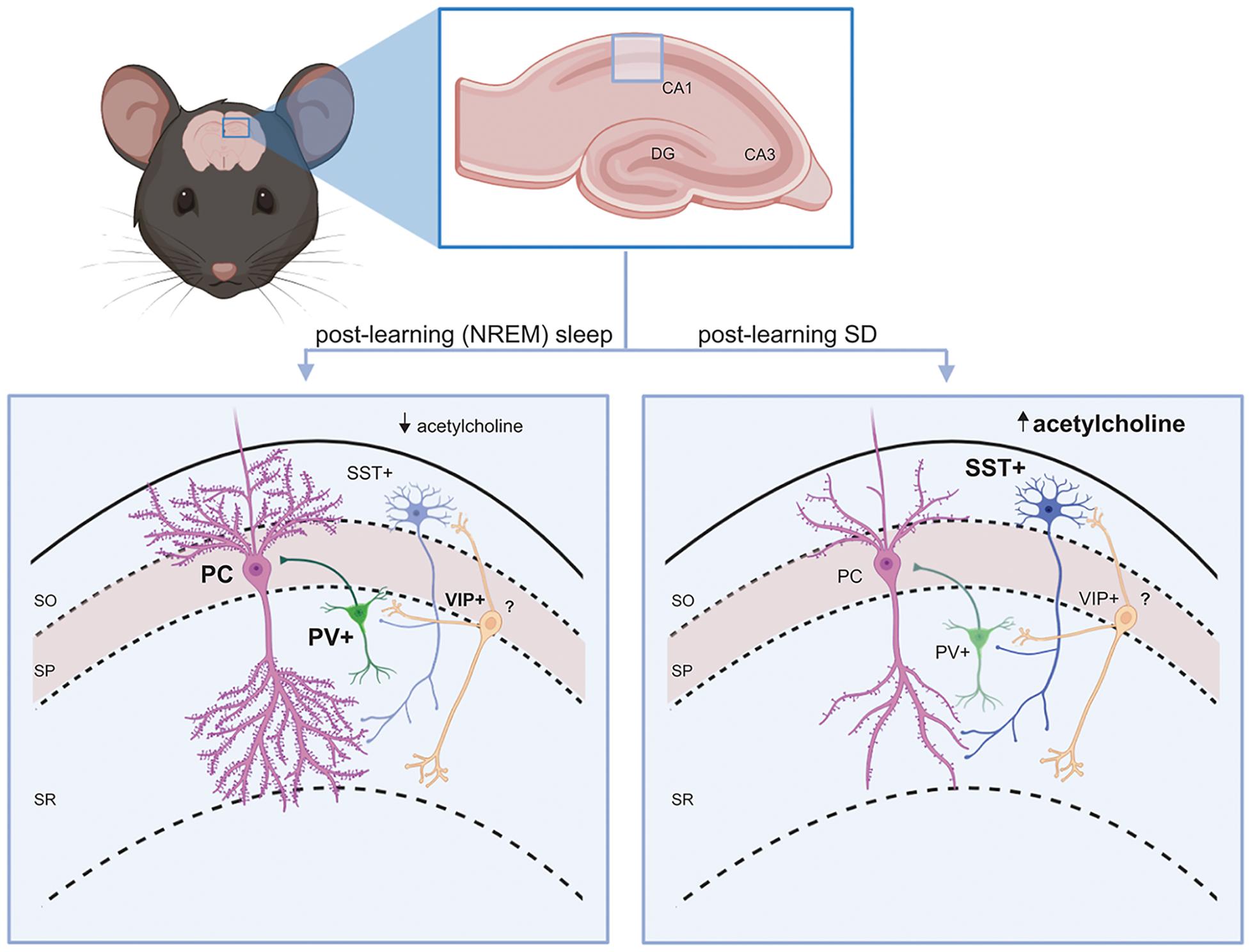
Which atom contains exactly 15 protons? An ion with 5 protons, 6 neutrons, and a charge of 3+ has an atomic number of a.
Which Atom Contains Exactly 15 Protons. Each n atom has 7 protons, so each n2 molecule has 14 protons. An ion with 5 protons, 6 neutrons, and a charge of 3+ has an atomic number of a. Which atom contains exactly 15 protons? Get an answer to your question “which atom contains exactly 15 protons.” in 📙 physics if there is no answer or all answers are wrong, use a search bar and try to find the answer among similar questions.
 Which Atom Contains Exactly 15 Protons? - Quora From quora.com
Which Atom Contains Exactly 15 Protons? - Quora From quora.com
Related Post Which Atom Contains Exactly 15 Protons? - Quora :
Refer to the periodic table for element 15 (phosphorus). If an atom contains exactly 79 protons, then it�s an atom of _____. 56 62 90 28 what is the total number of atoms represented in the formula cuso4. Each o atom has 8 protons, so each o2 molecule has 16 protons.
An atom of sulfur (s) contains 6 valence electrons.
An unknown substance, liquid x, is tested in the laboratory. 1 answer to which atom contains exactly 15 protons? For a given element, the atomic number indicates the number of _____. When alpha particles are used to bombard gold foil, most of the alpha particles pass through undeflected. 7) what is the mass number of an atom which contains 28 protons, 28 electrons, and 34 neutrons? 56 62 90 28 what is the total number of atoms represented in the formula cuso4.
 Source:
Source:
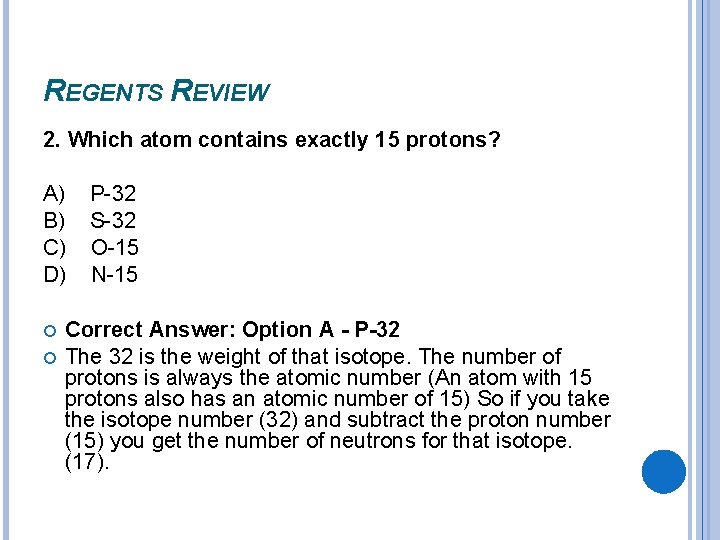
1 answer to which atom contains exactly 15 protons? Phosphorus has atomic number 15, so it contains exactly 15 protons. 7) what is the mass number of an atom which contains 28 protons, 28 electrons, and 34 neutrons?
 Source: slideplayer.com
Source: slideplayer.com
8 protons, 8 neutrons, 10 electrons 3. By referring to a periodic table or table of elements, we see that phosphorus (symbol p) has an atomic number of 15. Because phosphorus has atomic number 15.
 Source: slideplayer.com
Source: slideplayer.com
The chemical and physical test. Bezglasnaaz and 3 more users found this answer helpful. Compared to the charge and mass of a proton, an electron has.
 Source: slidetodoc.com
Source: slidetodoc.com
Which atom contains exactly 15 protons? An ion with 5 protons, 6 neutrons, and a charge of 3+ has an atomic number of a. Solution for which atom contains exactly 15 protons?
 Source: studylib.net
Source: studylib.net
An opposite charge and a smaller mass. 56 62 90 28 what is the total number of atoms represented in the formula cuso4. 2 📌📌📌 question which atom contains exactly 15 protons?
 Source: quora.com
Source: quora.com
An atom with 15 protons also has an atomic number of 15. Which atom contains exactly 15 protons? Because phosphorus has atomic number 15.
 Source: chegg.com
Source: chegg.com
- which atom contains exactly 15 protons? By referring to a periodic table or table of elements, we see that phosphorus (symbol p) has an atomic number of 15. An unknown substance, liquid x, is tested in the laboratory.
 Source: slideplayer.com
Source: slideplayer.com
Because phosphorus has atomic number 15. When alpha particles are used to bombard gold foil, most of the alpha particles pass through undeflected. An opposite charge and a smaller mass.
 Source: slideplayer.com
Source: slideplayer.com
B) 35 17 d) 36 the nucleus of an atom consists of 8 protons and. The atom which contains exactly 15 protons is phosphorus. Number of protons is determined by the atomic number of the element.

By referring to a periodic table or table of elements, we see that phosphorus (symbol p) has an atomic number of 15. 7) what is the mass number of an atom which contains 28 protons, 28 electrons, and 34 neutrons? Number of protons is determined by the atomic number of the element.
 Source: quora.com
Source: quora.com
An atom of carbon 14 contains. What is the mass number of an atom which contains 28 protons, 28 electrons, and 34 neutrons? 8 protons, 8 neutrons, 10 electrons 3.
 Source: studylib.net
Source: studylib.net
An atom with 15 protons also has an atomic number of 15. The negatively charged particles called electrons revolve around the centre of the nucleus. Which atom contains exactly 15 protons?
 Source: brainly.in
Source: brainly.in
8 protons, 10 neutrons, 10 electrons. 5) which atom contains exactly 15 protons? The atom has 2 neutrons.
 Source: slideplayer.com
Source: slideplayer.com
6 protons 8 neutrons 6 electrons. 56 62 90 28 what is the total number of atoms represented in the formula cuso4. An ion with 5 protons, 6 neutrons, and a charge of 3+ has an atomic number of a.
 Source: slideplayer.com
Source: slideplayer.com
Which atom contains exactly 15 protons? The chemical and physical test. Each o atom has 8 protons, so each o2 molecule has 16 protons.
 Source: studylib.net
Source: studylib.net
A proton has approximately the same mass as. 2 📌📌📌 question which atom contains exactly 15 protons? The chemical and physical test.
 Source: docsity.com
Source: docsity.com
And atomic number is equal to number of protons present in an atom inside the nucleus. For an atom to be electrically neutral, it must contain the same number of _____. When alpha particles are used to bombard gold foil, most of the alpha particles pass through undeflected.
 Source: studylib.net
Source: studylib.net
There are 5 protons so the atomic number is also 5. 5) which atom contains exactly 15 protons? An unknown substance, liquid x, is tested in the laboratory.
 Source: quora.com
Source: quora.com
The history of atomic structure and quantum mechanics dates back to the times of democritus, the man who first proposed that. 6) an ion with 5 protons, 6 neutrons, and a charge of 3+ has an atomic number of. 7) what is the mass number of an atom which contains 28 protons, 28 electrons, and 34 neutrons?
 Source: en.wikipedia.org
Source: en.wikipedia.org
8 protons, 8 neutrons, 10 electrons 3. If 75 % of isotopes of an element have a mass of 35.amu and 25.0 of the isotopes have a mass of 37 amu what is the atomic mass of the element. This result indicates that most of the volume of a gold atom consists of ____.
Also Read :