0.75 m c x 6 h x 21 o x 6. `(k_(f) h_(2)o = 1.86 k asked apr 1, 2020 in chemistry by chithrajain ( 84.1k points)
Which Aqueous Solution Has The Lowest Freezing Point. 0.01 molal nacl solution have minimum freezing point because its molality is more and it given two ions after dissociation of one molecule. Which aqueous solution has the smallest freezing point depression? Or to put into another way: 0.04 m urea [ (nh_2)_2c = o] 0.01 m agno_3 0.03 m cuso_4 which aqueous solution has the highest freezing point?
 Solved Which Aqueous Solution Has The Lowest Freezing Point? | Chegg.com From chegg.com
Solved Which Aqueous Solution Has The Lowest Freezing Point? | Chegg.com From chegg.com
Related Post Solved Which Aqueous Solution Has The Lowest Freezing Point? | Chegg.com :
Freezing point of a pure solvent depends on. Or to put into another way: A) al2 (so4)3 b) c6h12o6 c) ki d) c12h22o11. 4 0 o c.if the substance has normal molecular weight in benzene and is completely dissociated in water, into how many ions does it dissociate in water?
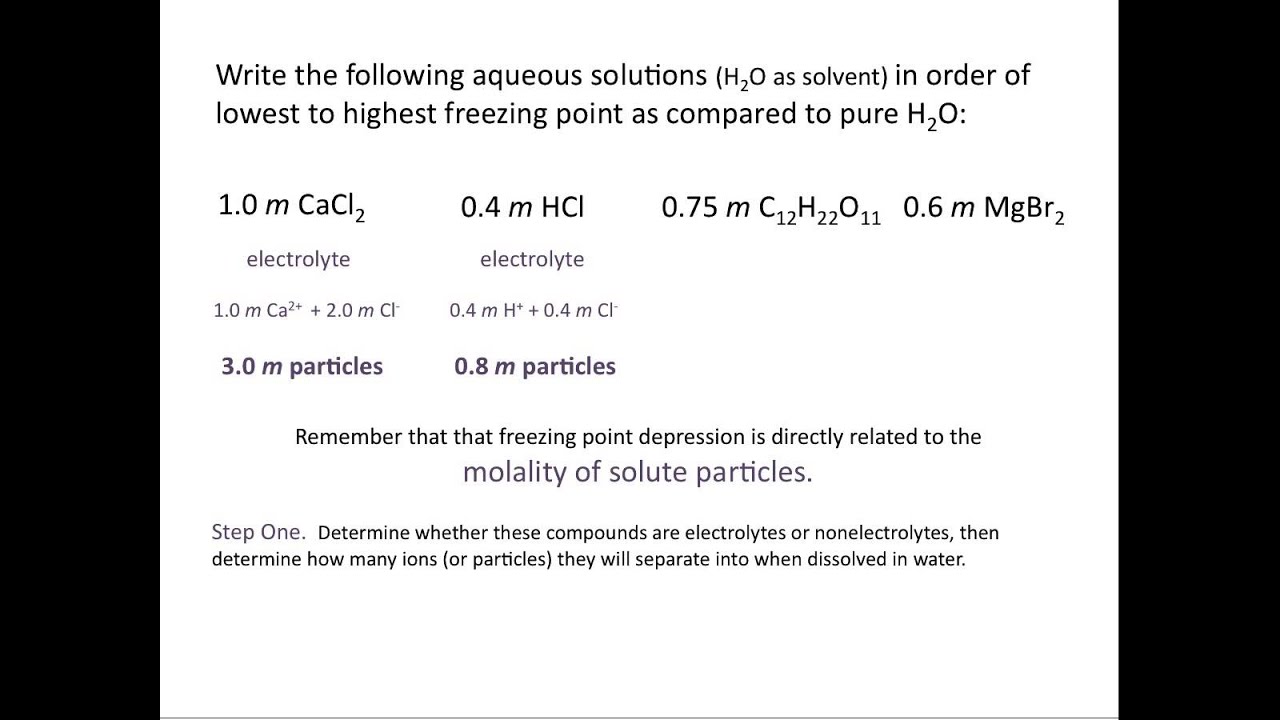
That is they depend upon the concentration of solute particles present.
0.04 m urea [ (nh_2)_2c = o] 0.01 m agno_3 0.03 m cuso_4 which aqueous solution has the highest freezing point? 0.04 m urea [ (nh_2)_2c = o] 0.01 m agno_3 0.03 m. A certain mass of a substance, when dissolved in 100 g of c 6 h 6 , lowers the freezing point by 1. Pure water has a normal boiling point of 100c, and by adding a solute the boiling point is raised. Arrange the following aqueous solutions in order of increasing freezing points (lowest to highest temperature): 1) 10 g of ki dissolved in 100 g of water:
 Source: youtube.com
Source: youtube.com
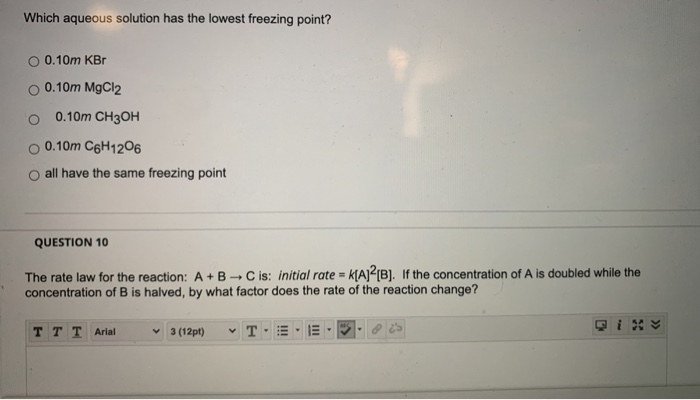
Which one of the following 0.06 m aqueous solutions has lowest freezing point ? The freezing point depression of a 0.01m solution of nacl is greater than the freezing point depression of a 0.01m glucose solution. 0.12 m ca(no 3) 2.
 Source: youtube.com
Source: youtube.com
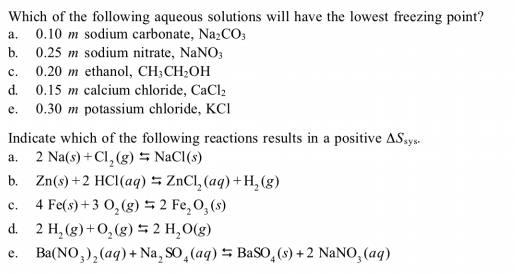
(a) 0.2 m ca(no3)2 (c) 0.2 m mgso4 (b) 0.2 m ch3oh (d) 0.2 m k3po4 18. This again is ionic but we get two moles of anions per mol of compound in. The lowest boiling point at 1 atm pressure is 0.1 m kcl.
 Source: chegg.com
Source: chegg.com
2 8 o c.the same mass of solute dissolved in 100 g of water lowers the freezing point by 1. `(k_(f) h_(2)o = 1.86 k asked apr 1, 2020 in chemistry by chithrajain ( 84.1k points) So it has the maximum freezing point.
 Source: doubtnut.com
Source: doubtnut.com
0.01 molal nacl solution have minimum freezing point because its molality is more and it given two ions after dissociation of one molecule. 0.5 m m g c l x 2. Therefore a 0.01m nacl solution has a lower freezing point than a 0.01m solution of glucose.
 Source: toppr.com
Source: toppr.com
The reasons why are dictated by quantum mechanics: Arrange the following aqueous solutions in order of increasing freezing points (lowest to highest temperature): 1.0% by mass kcl and 10% by mass of glucose is:
 Source: clutchprep.com
Source: clutchprep.com
A) al2 (so4)3 b) c6h12o6 c) ki d) c12h22o11. 4) 40 g of ki dissolved in 200 g of water. The material with the lowest freezing point is helium.
 Source: chegg.com
Source: chegg.com
Under typical pressures, it does not freeze at all, even at temperatures approaching absolute zero. Which of the following solutions will have minimum freezing point? So the lowest freezing point has the aqueous solution of nacl.

Arrange the following aqueous solutions in order of decreasing freezing points (lowest to highest temperature): Boiling point elevation (and freezing point depression) are colligative properties. 2 8 o c.the same mass of solute dissolved in 100 g of water lowers the freezing point by 1.
 Source: doubtnut.com
Source: doubtnut.com
0.01 molal nacl solution have minimum freezing point because its molality is more and it given two ions after dissociation of one molecule. The lowest boiling point at 1 atm pressure is 0.1 m kcl. 2 8 o c.the same mass of solute dissolved in 100 g of water lowers the freezing point by 1.
 Source: chegg.com
Source: chegg.com
(a) 0.2 m ca(no3)2 (c) 0.2 m mgso4 (b) 0.2 m ch3oh (d) 0.2 m k3po4 18. 4) 40 g of ki dissolved in 200 g of water. 0.75 m effective particle concentration.
 Source: chegg.com
Source: chegg.com
So, most particles of the solute is going to be in the solution of nacl, so the lowest freezing point has the aqueous solution of nacl. What causes depression in freezing point class 12? 0.5 m m g c l x 2.
 Source: bartleby.com
Source: bartleby.com
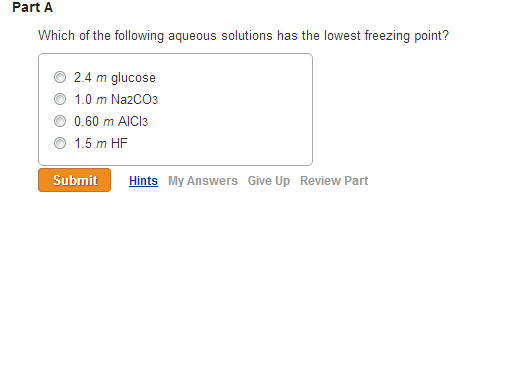
The freezing point of an aqueous solution of urea (h 2 nconh 2) at normal pressure (1 atm) is 273.06 k. As the depression is maximum, the solution will have lowest freezing point. Which aqueous solution has lowest freezing point?
 Source: bartleby.com
Source: bartleby.com
The lowest boiling point at 1 atm pressure is 0.1 m kcl. So it has the maximum freezing point. 0.75 m effective particle concentration.
 Source: chegg.com
Source: chegg.com
What causes depression in freezing point class 12? Antifreeze is an aqueous solution of ethylene glycol in water. That is they depend upon the concentration of solute particles present.
 Source: chegg.com
Source: chegg.com
- 10 g of ki dissolved in 100 g of water: Chemistry the boiling point of an aqueous solution is 102.48 °c. The material with the lowest freezing point is helium.
 Source: chegg.com
Source: chegg.com
The freezing point of an aqueous solution of urea (h 2 nconh 2) at normal pressure (1 atm) is 273.06 k. Which of the following aqueous solutions has the lowest freezing point? (a) 0.2 m ca(no3)2 (c) 0.2 m mgso4 (b) 0.2 m ch3oh (d) 0.2 m k3po4 18.
 Source: chegg.com
Source: chegg.com
Solved which aqueous solution has the lowest freezing point? 2 8 o c.the same mass of solute dissolved in 100 g of water lowers the freezing point by 1. Which has the lowest freezing point at 1 atm pressure?
 Source: chegg.com
Source: chegg.com
0.5 m m g c l x 2. A 0.1 m aqueous solution is made of each of the substances listed. The freezing point of an aqueous solution of urea (h 2 nconh 2) at normal pressure (1 atm) is 273.06 k.
 Source: chegg.com
Source: chegg.com
As the depression is maximum, the solution will have lowest freezing point. Remember, the greater the concentration of particles, the lower the freezing point will be. 0.1mcai2 will have the lowest freezing point, followed by 0.1mnacl, and the highest of the three solutions will be 0.1mc6h12o6, but all three of them will have a.
 Source: chegg.com
Source: chegg.com
Freezing point depression of a solution, when a nonvolatile solute is dissolved in a solvent, is a colligative property, which means that it depends on the number of particles of solute dissolved. A) al2 (so4)3 b) c6h12o6 c) ki d) c12h22o11. `(k_(f) h_(2)o = 1.86 k asked apr 1, 2020 in chemistry by chithrajain ( 84.1k points)
Also Read :





