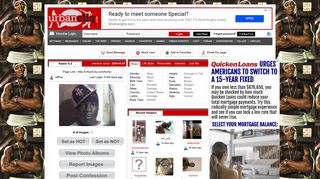
None of the above (all have the same boiling point) Which aqueous solutions will exhibit highest boiling point solutions which one of the following aqueous solutions will exhibit highest boiling point?
Which Aqueous Solution Has The Highest Boiling Point. This discussion on which of the given aqueous solution has maximum boiling point? For more information about the boiling point, refer to the link: The only difference between these three solutions are there either van toff factor. Likewise, people ask, which aqueous solution has highest boiling point?
 Which Of The Following Aqueous Solutions Should Have The Highest Boiling Point? From toppr.com
Which Of The Following Aqueous Solutions Should Have The Highest Boiling Point? From toppr.com
Related Post Which Of The Following Aqueous Solutions Should Have The Highest Boiling Point? :
1 % solution of z n s o x 4. (a) which solution is expected to have the higher boiling point: The ph of the solution is 1 % solution of ( n h x 2) x 2 c o.
Of the compounds below, a 0.1 m aqueous solution of _____ will have the highest ph.
Hence, 1.0 m na2st4 has highest value of boiling point. Which solution has the lowest freezing point? 1 % solution of n a c l. (a) which solution is expected to have the higher boiling point: Which of the following compounds has the highest boiling point; Ok, so the answer, um, is in a to four, it will have the highest boiling point on.
 Source: toppr.com
Source: toppr.com
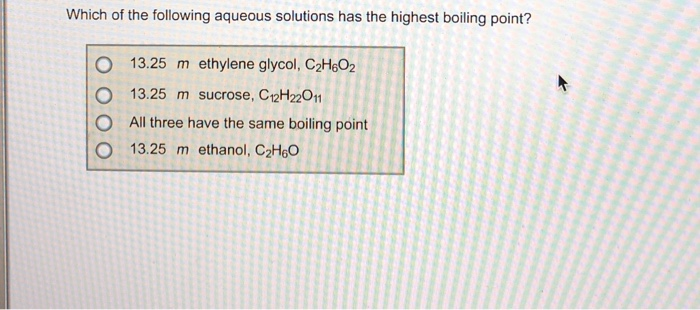
Which of the following aqueous solutions would have the highest boiling point Which of these aqueous solutions has the highest boiling point? (b) as we know greater the value of van’t hoff factor higher will be the elevation in boiling point and hence higher will be the boiling point of solution.
 Source: toppr.com
Source: toppr.com
1 % solution of ( n h x 2) x 2 c o. The ph of the solution is 1 % solution of n a c l.
 Source: youtube.com
Source: youtube.com
For more information about the boiling point, refer to the link: Which one of the following aqueous solutions will exhibit highest boiling point (a) 0.01 m na2so4 (b) 0.015 m glucose (c) 0.015 m urea (d) 0.01 m kno 3 2 see answers advertisement advertisement anjali1313 anjali1313 (a) 0.01 m na2so4 has highest boiling point because its vant hoff factor i.e i = 3 and i is directly proportional to boiling point Hence, 1.0 m na2st4 has highest value of boiling point.
 Source: toppr.com
Source: toppr.com
Since calcium nitrate has the highest van�t hoff factor, the boiling point of the compound has been the highest. Of the compounds below, a 0.1 m aqueous solution of _____ will have the highest ph. 1 % solution of ( n h x 2) x 2 c o.
 Source: toppr.com
Source: toppr.com
Which aqueous solution has the highest boiling point? The ph of the solution is Why does 0.1 m kcl have a higher boiling point?
 Source: clutchprep.com
Source: clutchprep.com
Of the compounds below, a 0.1 m aqueous solution of _____ will have the highest ph. Which of the following aqueous solutions would have the highest boiling point I think that the answer is d, the all have the same boiling point.
 Source: chegg.com
Source: chegg.com
(a) 1m glucose solution has highest freezing point because it has lowest δtf. Higher δt will have a higher boiling point. None of the above (all have the same boiling point)
 Source: slideplayer.com
Source: slideplayer.com
High value for the van�t hoff factor, high boiling point). Which one of the following aqueous solutions will exhibit highest boiling point (a) 0.01 m na2so4 (b) 0.015 m glucose (c) 0.015 m urea (d) 0.01 m kno 3 2 see answers advertisement advertisement anjali1313 anjali1313 (a) 0.01 m na2so4 has highest boiling point because its vant hoff factor i.e i = 3 and i is directly proportional to boiling point They all have the same boiling point.

The mole fraction of substance a is 0.35. (b) as we know greater the value of van�t hoff factor higher will be the elevation in boiling point and hence higher will be the boiling point of solution. Hence, 1.0 m na2st4 has highest value of boiling point.
 Source: chegg.com
Source: chegg.com
Which of the following compounds has the highest boiling point; Likewise, people ask, which aqueous solution has highest boiling point? As the pressure increases on a solution containing a gaseous solute, the solubility of the gas increases.
 Source: youtube.com
Source: youtube.com
Which of these aqueous solutions has the highest boiling point? 0.1 molal aqueous solutions of kcl will have higher boiling point because it is an electrolyte solute and on addition to the solvent it will give 2 ions and thus the no. I know that more is the van ’t hoff factor more will be the elevation in boiling point and hence the solution will have more boiling point.
 Source: numerade.com
Source: numerade.com
At higher pressures, there are more gas molecules above the solution, which are able to enter and dissolve into the solution. So fate has the highest factor, and so therefore, it will have the highest boiling point elevation and have the highest blowing. (1) 0.11 m kbr (2) 0.12 m cacl2 (3) 0.13 m nh4no3 (4) 0.14 m hcl (5) 0.15 m c6h12o6

Higher δt will have a higher boiling point. A solution contains a mixture of substance a and substance b, both of which are volatile. Which aqueous solution has the highest boiling point?

(b) as we know greater the value of van�t hoff factor higher will be the elevation in boiling point and hence higher will be the boiling point of solution. Likewise, people ask, which aqueous solution has highest boiling point? For more information about the boiling point, refer to the link:
 Source: sahay.guru
Source: sahay.guru
[kb= 0.512] (a) 1.25 m c6h12o6 (b) 1.25 m kno3 (c) 1.25 m ca(no3)2 (d) they all have the same boiling point. None of the above (all have the same boiling point) Thus, na3po4 has the highest boiling point.
 Source: chegg.com
Source: chegg.com
The aqueous solution with the highest boiling point has been. $0.20 \math… 04:37 rank the following aqueous solutions in order of increasing (a) osmotic pres… = boiling point elevation constant, m = molality.
 Source: studylib.net
Source: studylib.net
They all have the same boiling point. (a) 1m glucose solution has highest freezing point because it has lowest δtf. Since the al(no3)3 produces the greatest concentration of solute particles, it will raise the boiling point of the water to the greatest extent.
 Source: youtube.com
Source: youtube.com
Which of the following compounds has the highest boiling point; An aqueous solution contains 0.100 m naoh at 25.0 °c. Hence, 1.0 m na2st4 has highest value of boiling point.

1 % solution of z n s o x 4. Why does 0.1 m kcl have a higher boiling point? = boiling point elevation constant, m = molality.
 Source: youtube.com
Source: youtube.com
×m, where i = van hoff�s factor, k b. Of moles increases and boiling point elevation will be more. Which of these aqueous solutions has the highest boiling point?
Also Read :