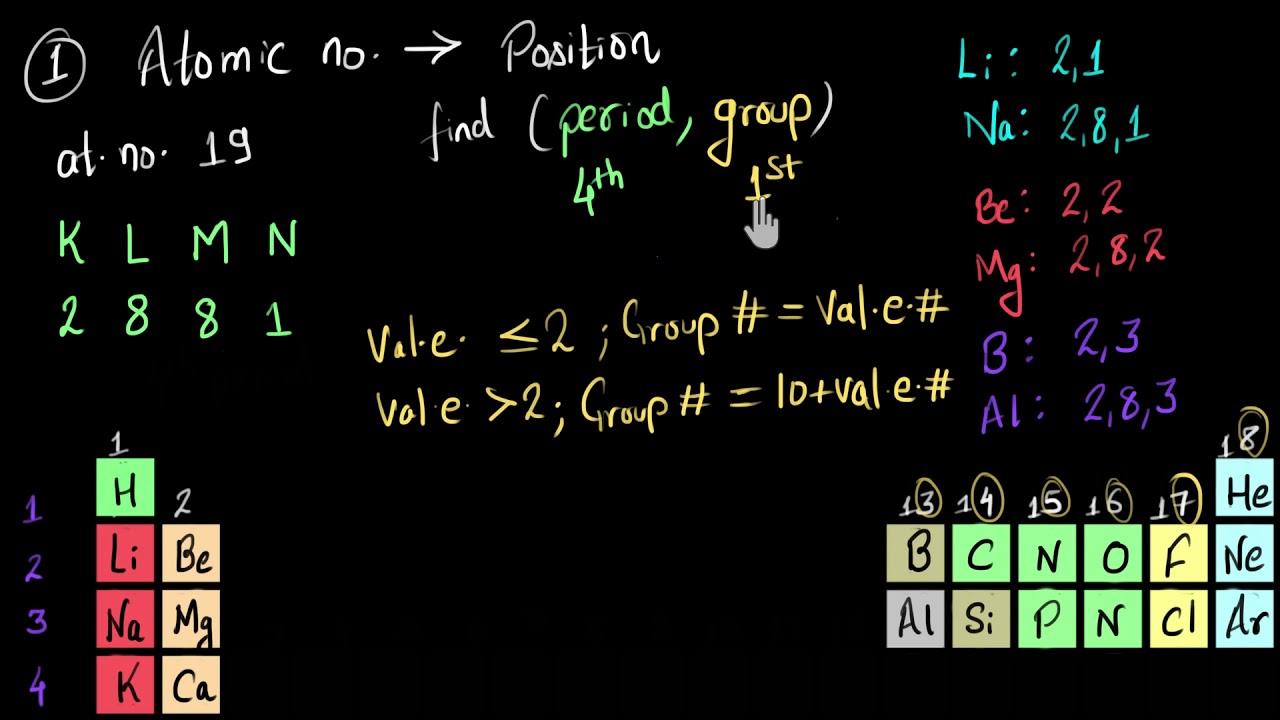
The period of an element corresponds to the principal quantum number of the valence shell. The atomic number of oganesson is 118.
What Predicts The Element To Which An Atom Belongs. What scientific concept do you need to know in order to solve this problem? Others are abbreviations of the name in another language. Because of their placement in the periodic table properties of several yet to be discovered elements like scandium, gallium and germanium could be predicted. Oganesson is a chemical element of unknown appearance and belongs to the group of transactinides.
 Main-Group Element - Wikipedia From en.wikipedia.org
Main-Group Element - Wikipedia From en.wikipedia.org
Related Post Main-Group Element - Wikipedia :
Bromine being halogen belongs to group 17 with a valence of 1. It is an extremely radioactive synthetic element.the element is named after the flerov laboratory of nuclear reactions of the joint institute for nuclear research in dubna, russia, where the element was discovered in 1998.the name of the laboratory, in turn, honours the russian. Atomic weight of element = n × (atomic weight of one hydrogen atom) atomic weight of h = 1 where n = number of hydrogen atom = 1, 2, 3,. This is approximately the sum of the number of protons and neutrons in the nucleus.
The symbols for several common elements and their atoms are listed in table 4.
Our tutors have indicated that to solve this problem you will need to apply the subatomic particles concept. The atomic number is the number of protons in the nucleus of an atom. The period of an element corresponds to the principal quantum number of the valence shell. Some symbols are derived from the common name of the element; The atomic number of an element represents the number of electrons or protons present in the atom of the element. It is defined as being the charge that an atom would have if all bonds were ionic.
 Source: courses.lumenlearning.com
Source: courses.lumenlearning.com
Hence the formula of the compound formed would be al2s3. The atomic mass of the middle element is equal to the arithmetic mean of the atomic masses of the other two elements. Atomic weight of element = n × (atomic weight of one hydrogen atom) atomic weight of h = 1 where n = number of hydrogen atom = 1, 2, 3,.

When electrons are removed in succession from an element, the transition from removing valence electrons to removing core electrons results in a large jump in ionization energy. Element with atomic number 12 is magnesium.the electronic configuration for mg= 1s 2 ,2s 2 2p element with atomic number 12 is magnesium.the electronic configuration for mg= 1s 2 ,2s 2 2p 6 element with atomic number 12 is magnesium.the electronic configuration for mg= 1s 2 ,2s 2 2p 6 ,3s element with atomic number 12 is magnesium.the electronic. It is defined as being the charge that an atom would have if all bonds were ionic.

What predicts the element to which an atom belongs? Mendeleev gave more weightage to similarities in properties of elements rather than atomic masses. Others are abbreviations of the name in another language.

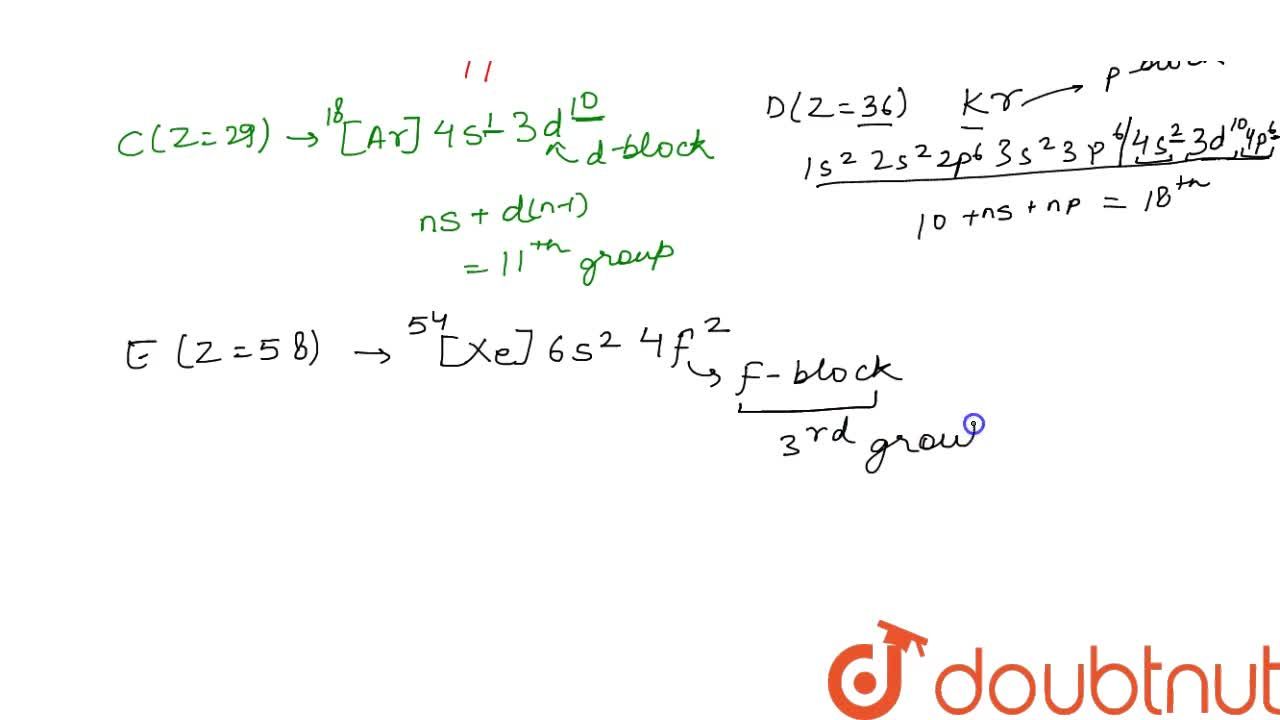
B) for p block elements,group number is equal to 10+number of electrons in the valence shell. The atomic number is the number of protons in the nucleus of an atom. Flerovium is a superheavy artificial chemical element with the symbol fl and atomic number 114.
 Source: doubtnut.com
Source: doubtnut.com
B) for p block elements,group number is equal to 10+number of electrons in the valence shell. A)for s block elements ,group number is equal to the number of valence electrons. The basis of this prediction is a rule known as the aufbau principle, which assumes that electrons are added to an atom, one at a time, starting with the lowest energy orbital, until all of the electrons have been placed in an appropriate orbital.
 Source: khanacademy.org
Source: khanacademy.org
The block of an element corresponds to the type of orbital which receive the last electron. It is an extremely radioactive synthetic element.the element is named after the flerov laboratory of nuclear reactions of the joint institute for nuclear research in dubna, russia, where the element was discovered in 1998.the name of the laboratory, in turn, honours the russian. Some symbols are derived from the common name of the element;
 Source: youtube.com
Source: youtube.com
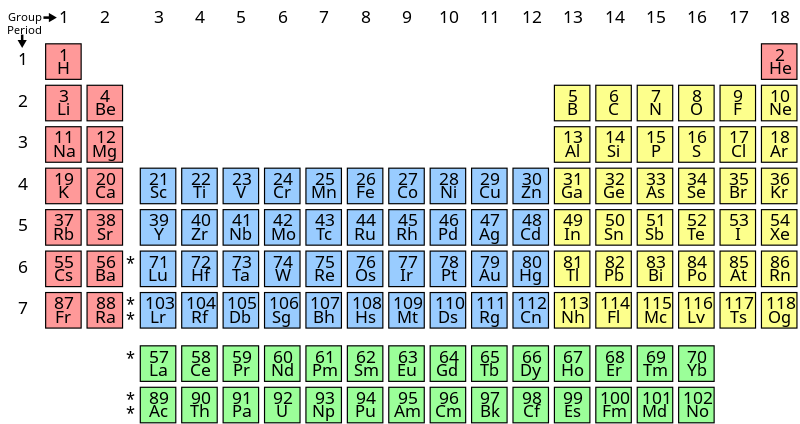
Elements in the same group have similar properties. This is approximately the sum of the number of protons and neutrons in the nucleus. To learn about the modern periodic table of elements and its significance and also, learn about the manner in which the elements are classified across the modern periodic table, visit byju’s for more information.

Some symbols are derived from the common name of the element; Of electrons in n orbital=2+1=3 hence, the element belongs to 3rd group. (a) silicon belongs to group 14 with a valence of 4.

Oganesson is a synthetic chemical element with the symbol og and atomic number 118. (b) the atomic weights of all. Oganesson is a synthetic chemical element with the symbol og and atomic number 118.
 Source: en.wikipedia.org
Source: en.wikipedia.org
When electrons are removed in succession from an element, the transition from removing valence electrons to removing core electrons results in a large jump in ionization energy. It is always a whole number greater than zero when used to identify an element. What scientific concept do you need to know in order to solve this problem?
 Source: en.wikipedia.org
Source: en.wikipedia.org
Oganesson is a chemical element of unknown appearance and belongs to the group of transactinides. Because of their placement in the periodic table properties of several yet to be discovered elements like scandium, gallium and germanium could be predicted. Sulphur belongs to group 16 elements with a valence of 1.
 Source: issuu.com
Source: issuu.com
Get 20% off grade+ yearly subscription → Others are abbreviations of the name in another language. What predicts the element to which an atom belongs?

(a) silicon belongs to group 14 with a valence of 4. The atomic mass of the middle element is equal to the arithmetic mean of the atomic masses of the other two elements. Atomic weight of element = n × (atomic weight of one hydrogen atom) atomic weight of h = 1 where n = number of hydrogen atom = 1, 2, 3,.
 Source: courses.lumenlearning.com
Source: courses.lumenlearning.com
What predicts the element to which an atom belongs? Atomic weight of element = n × (atomic weight of one hydrogen atom) atomic weight of h = 1 where n = number of hydrogen atom = 1, 2, 3,. Oxygen, nitrogen, hydrogen, carbon the four most common elements, comprising 96% of the body�s mass are _______.
 Source: toppr.com
Source: toppr.com
By looking for this large jump in energy, we can determine how many valence electrons an element has, which in turn can help us identify the element. When electrons are removed in succession from an element, the transition from removing valence electrons to removing core electrons results in a large jump in ionization energy. What predicts the element to which an atom belongs?
 Source: clutchprep.com
Source: clutchprep.com
This topic is discussed across several sections of this book. Where more than one isotope exists, the value given is the abundance weighted average. The group of an element is predicted from the number of.
 Source: studocu.com
Source: studocu.com
What predicts the element to which an atom belongs? The basis of this prediction is a rule known as the aufbau principle, which assumes that electrons are added to an atom, one at a time, starting with the lowest energy orbital, until all of the electrons have been placed in an appropriate orbital. Using both these concepts, we can determine the electronic configuration of the given element.
 Source: courses.lumenlearning.com
Source: courses.lumenlearning.com
B) for p block elements,group number is equal to 10+number of electrons in the valence shell. It is always a whole number greater than zero when used to identify an element. If you don’t have a chart, you can still find the electron configuration.
 Source: issuu.com
Source: issuu.com
A)for s block elements ,group number is equal to the number of valence electrons. According to dobereiner’s law of triads, when elements are arranged in increasing order of their atomic masses, a group of elements with similar chemical properties is obtained. It is an extremely radioactive synthetic element.the element is named after the flerov laboratory of nuclear reactions of the joint institute for nuclear research in dubna, russia, where the element was discovered in 1998.the name of the laboratory, in turn, honours the russian.
 Source: doubtnut.com
Source: doubtnut.com
Others are abbreviations of the name in another language. The first periodic table of elements. The period of an element corresponds to the principal quantum number of the valence shell.
Also Read :




