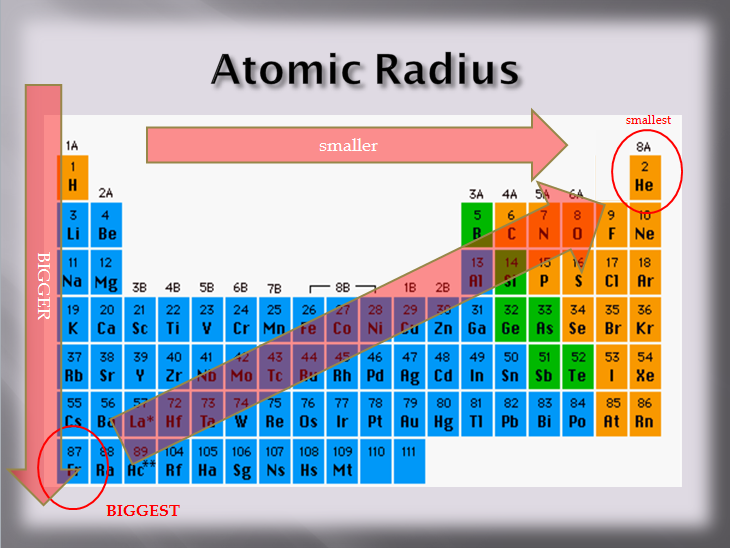
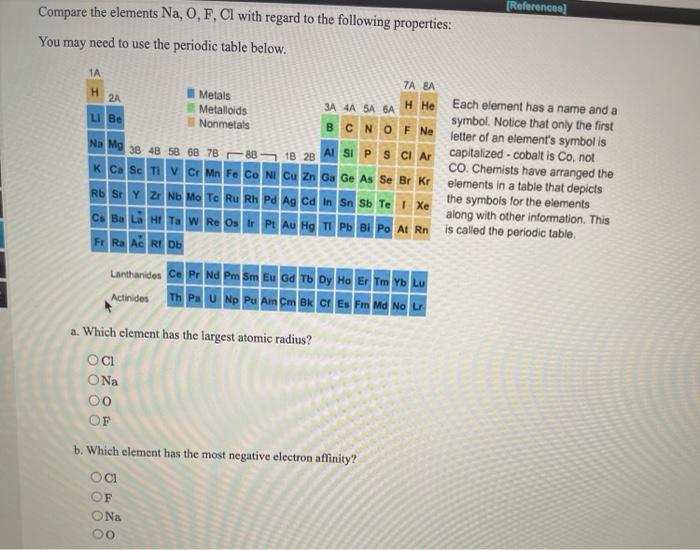
Thus, helium is the smallest element, and francium is the largest. Which atom or ion has the smallest radius?
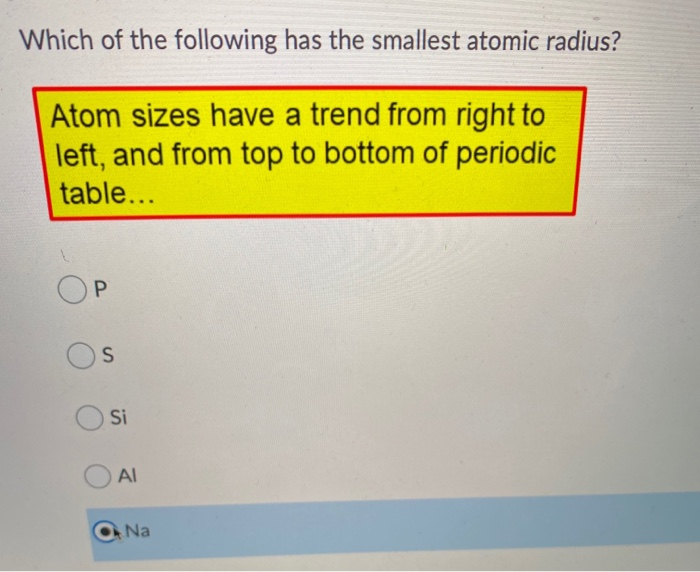
Of The Following Which Atom Has The Smallest Atomic Radius. Ionic radius of mn3+ will be largest. One such trend involves the atomic radius for these elements. Atomic radius decreases as you move across a period from left to right and decreases as you move up a group from bottom to top. A) na b) al c) n d) f.
 8.2 - Periodic Trends - Physical Science From physicalsciencetext.weebly.com
8.2 - Periodic Trends - Physical Science From physicalsciencetext.weebly.com
Related Post 8.2 - Periodic Trends - Physical Science :
Which element has the bigger radius? Thus, helium is the smallest element, and francium is the largest. This is due to trends in the periodic table, and the effective nuclear charge that holds the valence electrons close to the nucleus. Atomic radius generally increases as we move _____.
A) br b) as c) ca d) k.
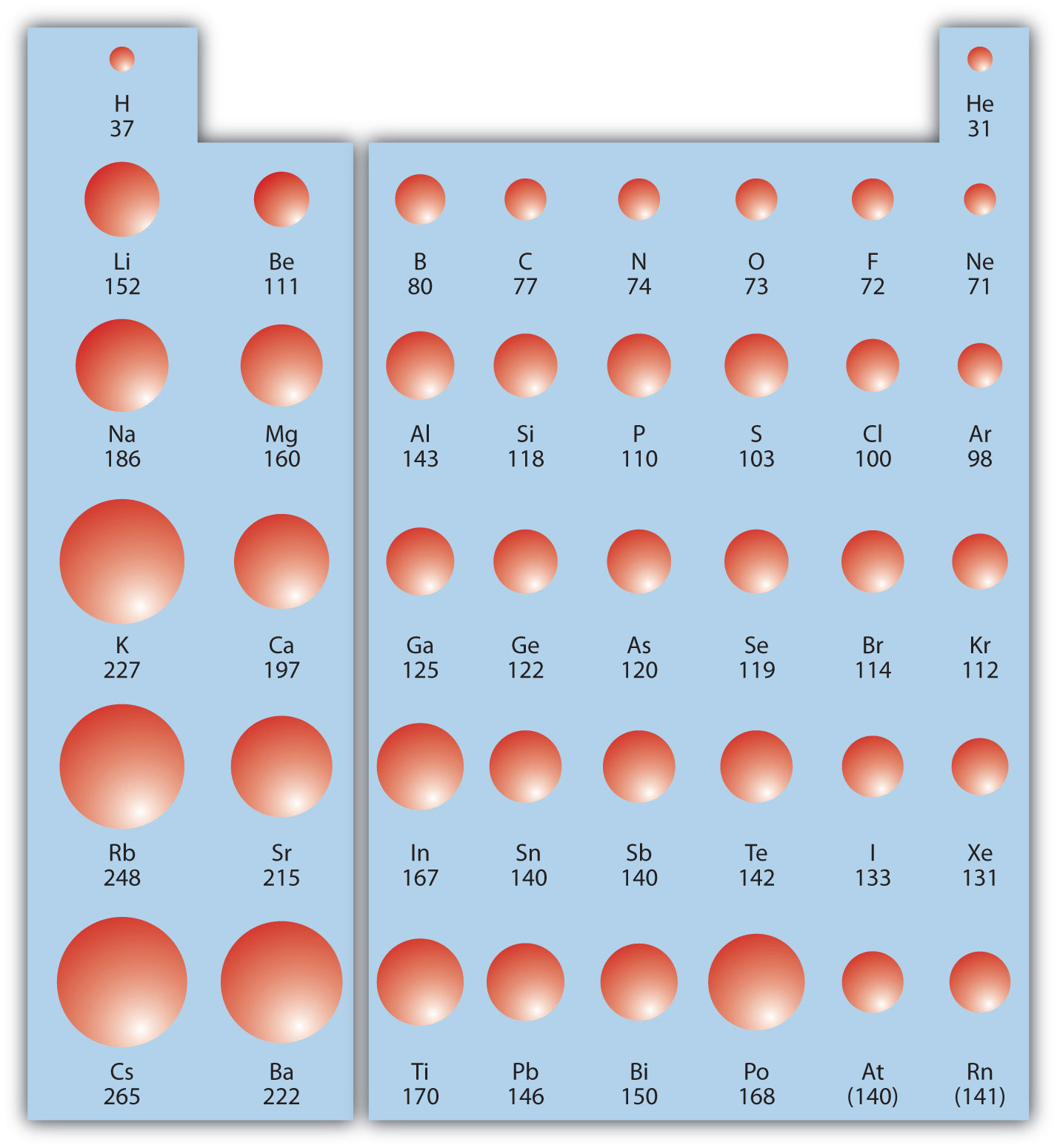
Heliumas can be seen in the figures below, the atomic radius increases from top to bottom in a group, and decreases from left to right across a period. Rank the following atoms in order of the largest to smallest atomic radius: This homework question can be solved by thinking about how the charges react to each other. The atomic mass of titanium is 47.88 atomic mass units. Atomic radius decreases as you move across a period from left to right and decreases as you move up a group from bottom to top. This is due to trends in the periodic table, and the effective nuclear charge that holds the valence electrons close to the nucleus.
 Source: angelo.edu
Source: angelo.edu
Which atom has the lowest first ionization energy? Which of the following correctly lists the five atoms in order of the five atoms in order of increasing size (smallest to largest?) Which of the following has the largest radius k na+ na k+?
 Source: chegg.com
Source: chegg.com
Which atom or ion has the smallest radius? Does al or al3+ have a larger radius? This homework question can be solved by thinking about how the charges react to each other.
 Source: chegg.com
Source: chegg.com
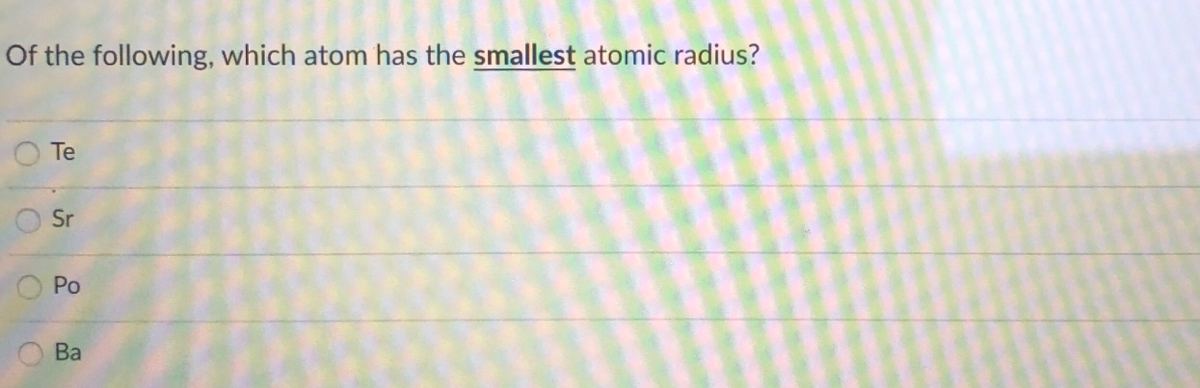
Of the following, which atom has the smallest atomic radius? As the nuclear charge increases the attractive force between nucleus and electrons increases. A) na b) ba c) ca d) cs.

Which atom or ion has the smallest radius? Which element has the bigger radius? Helium has the smallest atomic radius.
 Source: numerade.com
Source: numerade.com
Which has largest radius co 3? The first electron will circle that at some distance, making a h atom. The right most comer of perioder and it belongs to group 17 and we know that across the period aromir rodres decreases hence correct option is @ i lodre.
 Source: clutchprep.com
Source: clutchprep.com
The first electron will circle that at some distance, making a h atom. Which has largest radius co 3? In periods, atomic radius decreases from left to right.
 Source: blog.prepscholar.com
Source: blog.prepscholar.com
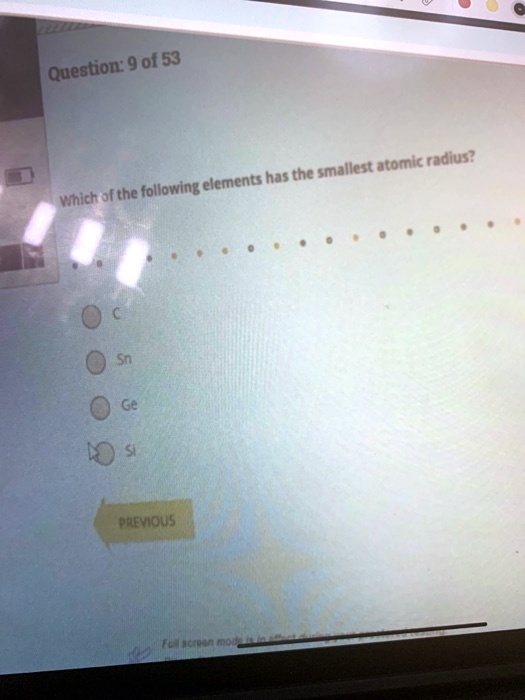
What is the atomic number of this atom? Question 16 of 25 0.0/ 4.0 points of the following, which atom has the smallest atomic radius? Ionic radius of mn3+ will be largest.
The h nucleus has a single positive charge. Rank the following atoms in order of the largest to smallest atomic radius: Of the following which gives the current order for the atomic radius for mg, na, p, si, and ar?
 Source: clutchprep.com
Source: clutchprep.com
Which of the following atoms has the smallest radius? Al3+ has the smallest size. G p po 16 6 sr 2 5 ba 2 6 te 16 5
 Source: bartleby.com
Source: bartleby.com
A) na b) al c) n d) f. What is the atomic number of this atom? Ionic radius of mn3+ will be largest.
 Source: physicalsciencetext.weebly.com
Source: physicalsciencetext.weebly.com
As the nuclear charge increases the attractive force between nucleus and electrons increases. Which atom or ion has the smallest radius? As the nuclear charge increases the attractive force between nucleus and electrons increases.
 Source: socratic.org
Source: socratic.org
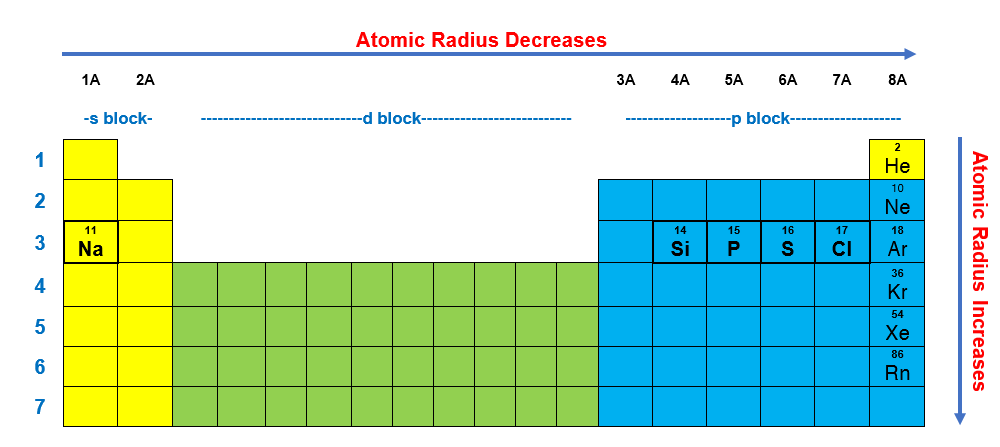
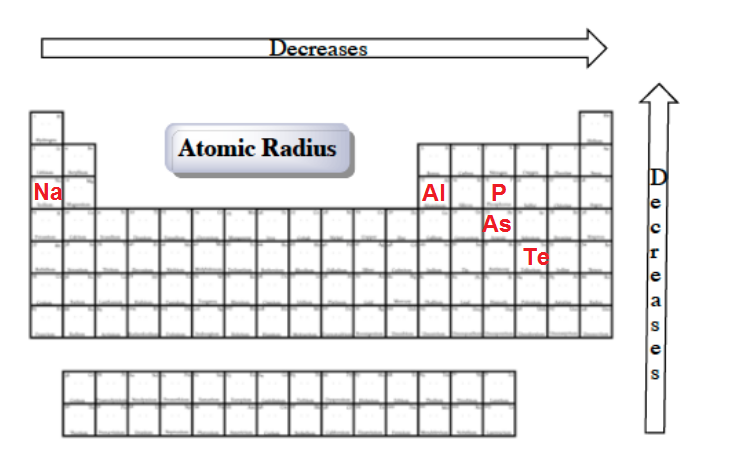
Al, p, cl, k k>al>p>cl below are data on the first four ionization energies for the fictitious element x Atomic radii vary in a predictable way across the periodic table. A) na b) ba c) ca d) cs.
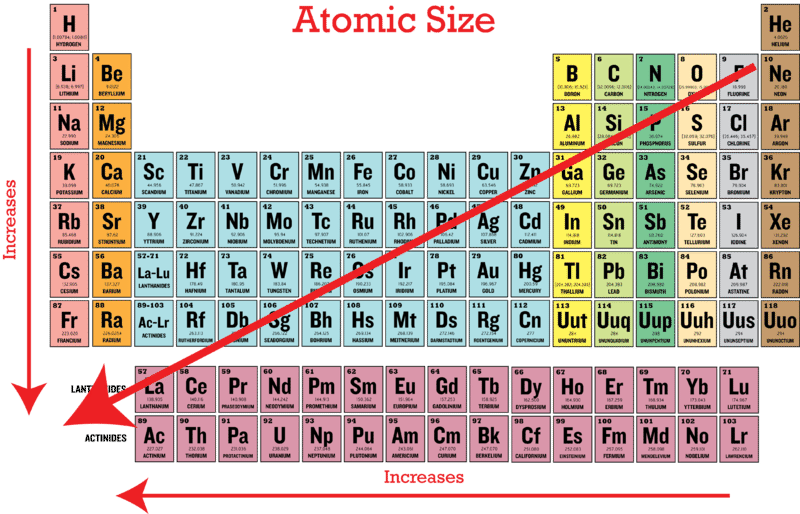
Ionic radius of mn3+ will be largest. As can be seen in the figures below, the atomic radius increases from top to bottom in a group, and decreases from left to right across a period. Al3+ has the smallest size.
 Source: youtube.com
Source: youtube.com
Because of the charge present on it. As can be seen in the figures below, the atomic radius increases from top to bottom in a group, and decreases from left to right across a period. A) br b) as c) ca d) k.

A) na b) ba c) ca d) cs. Al, p, cl, k k>al>p>cl below are data on the first four ionization energies for the fictitious element x The first electron will circle that at some distance, making a h atom.
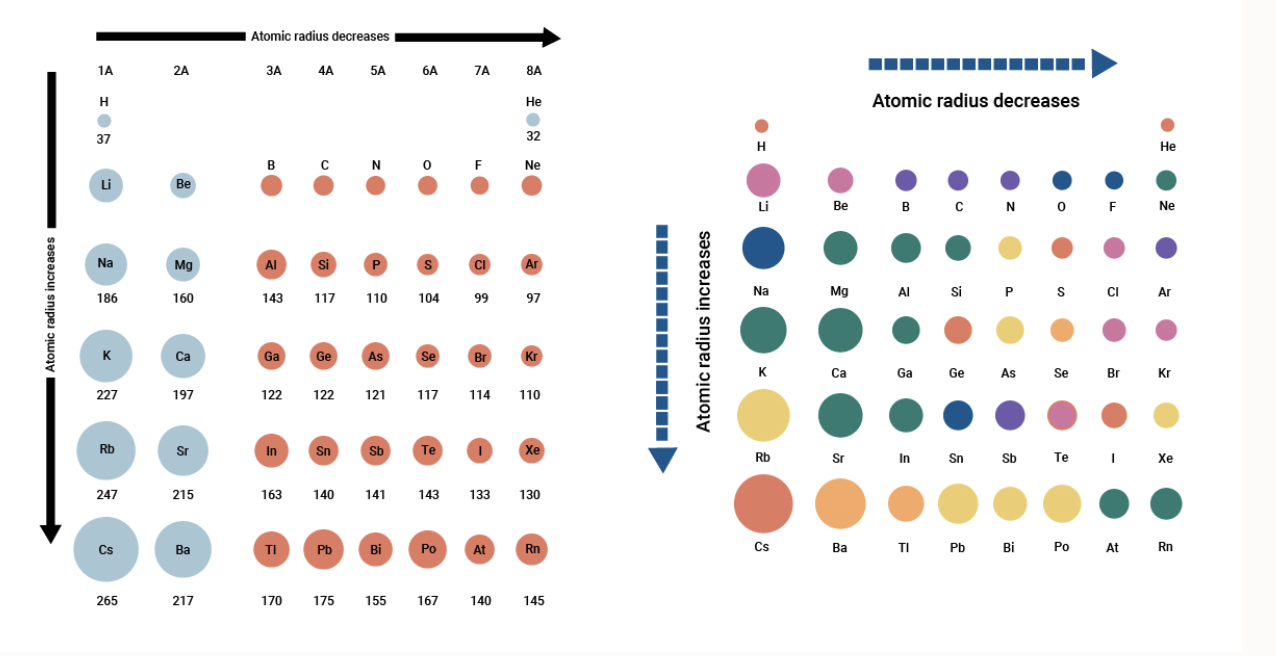 Source: breakingatom.com
Source: breakingatom.com
As the nuclear charge increases the attractive force between nucleus and electrons increases. A) rb b) si c) s d) o. Which of these elements has the smallest atomic radius apex answers?
 Source: quizlet.com
Source: quizlet.com
Thus, helium is the smallest element, and francium is the largest. Thus, helium is the smallest. A) na b) al c) n d) f.
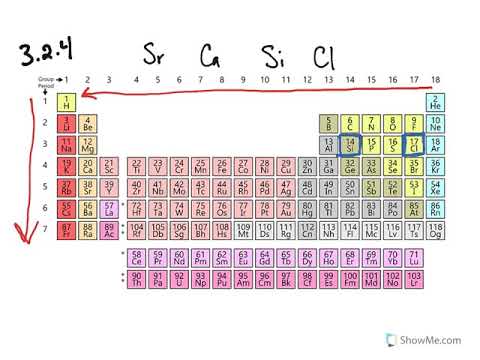 Source: chem.libretexts.org
Source: chem.libretexts.org
K+ has a larger atomic radius than na+. Question 16 of 25 0.0/ 4.0 points of the following, which atom has the smallest atomic radius? Which of the following correctly lists the five atoms in order of the five atoms in order of increasing size (smallest to largest?)
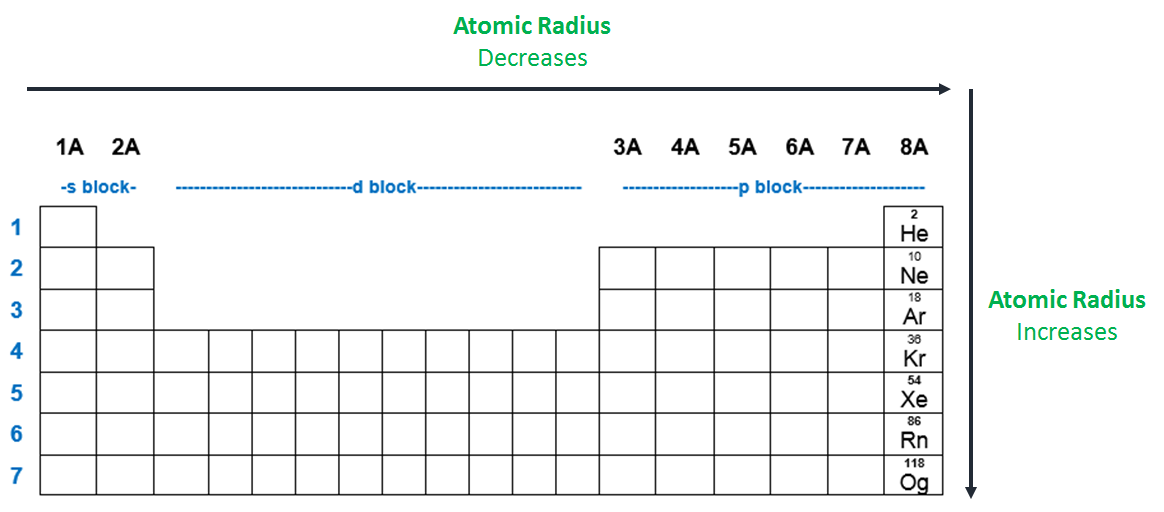 Source: clutchprep.com
Source: clutchprep.com
K+ has a larger atomic radius than na+. One such trend involves the atomic radius for these elements. Which of the following atoms has the smallest radius?
 Source: chegg.com
Source: chegg.com
Al, p, cl, k k>al>p>cl below are data on the first four ionization energies for the fictitious element x Heliumas can be seen in the figures below, the atomic radius increases from top to bottom in a group, and decreases from left to right across a period. Of the following which gives the current order for the atomic radius for mg, na, p, si, and ar?
Also Read :





