Of the following molecules, which has the largest dipole moment? Ch2cl2 and ch3cl will have a small resultant dipole because.
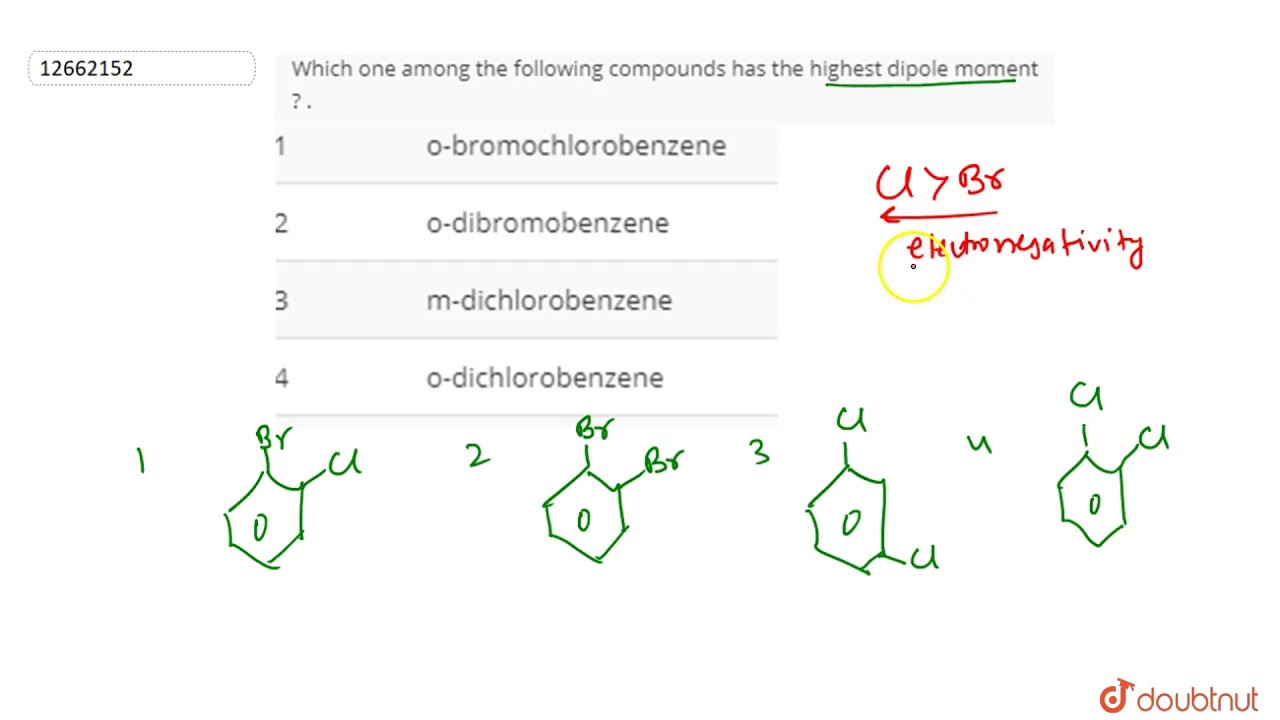
Of The Following Molecules Which Has The Largest Dipole Moment. In the structure it is clear that the two chlorine atoms present opposite to each other cancel each other’s dipole moments. Among the following, the molecule with the highest dipole moment is : C h ( c l) 3. Which bond angle (θ) would result in the maximum dipole moment for the triatomic molecule xy2?
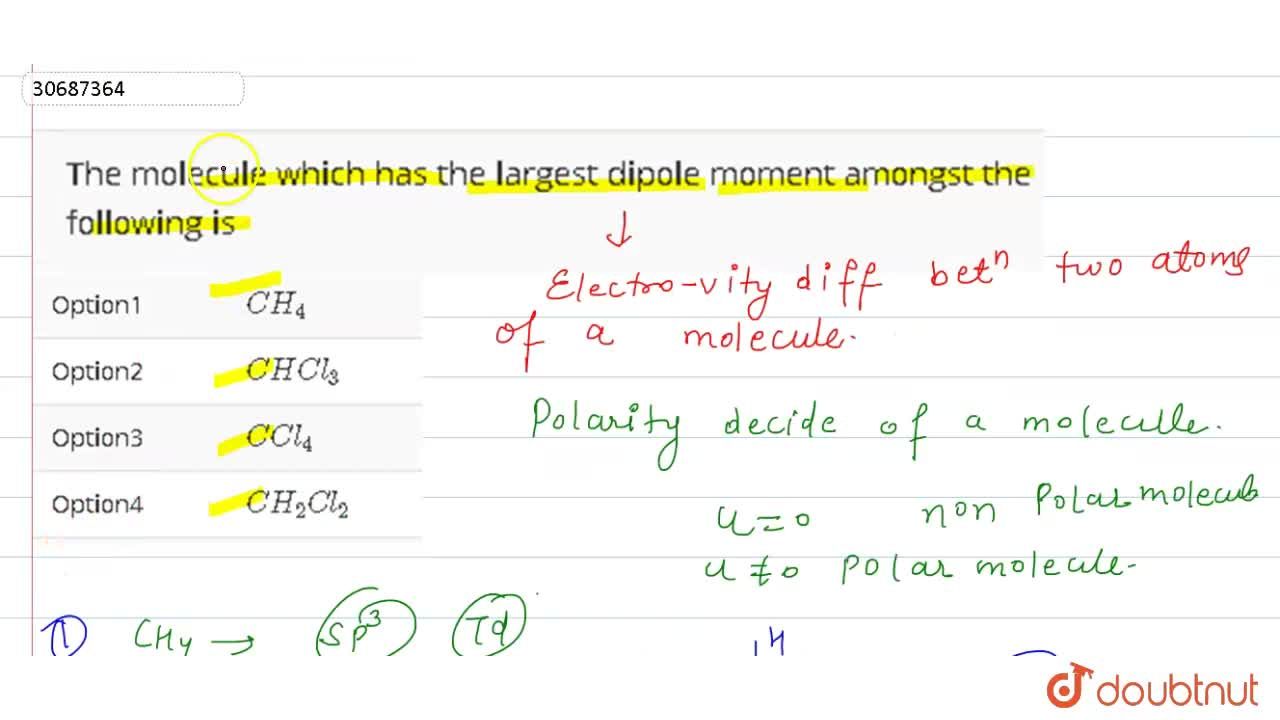 The Molecule Which Has The Largest Dipole Moment Amongst The Following Is From doubtnut.com
The Molecule Which Has The Largest Dipole Moment Amongst The Following Is From doubtnut.com
Related Post The Molecule Which Has The Largest Dipole Moment Amongst The Following Is :
(a) chl3 (b) ch4 (c) chcl3 (d) ccl4. Among the two and the compound having more dipole moment is nh_3. Which of the following molecules has the largest dipole moment? Hence, only the cl group contributes to the dipole moment of the molecule.
The dipole moment is defined by the charge separated multiplied by the distance (separation) between the charges.
This is because the individual dipole moments cancel out because of the symmetrical tetrahedral. Among the two and the compound having more dipole moment is nh_3. In the chloromethane molecule (ch 3 cl), chlorine is more electronegative than carbon, thus attracting the electrons in the c—cl bond toward itself. In the case of c h 3 c l, the dipole moment between c and h bond is 0. Which of the following species has the largest dipole moment (i.e., is the most polar)? Therefore, c h 2 c l 2 has the highest dipole moment amongst the above three molecules.
 Source: slideplayer.com
Source: slideplayer.com
Ch2cl2 and ch3cl will have a small resultant dipole because. Asked sep 30, 2016 in chemistry by trollcoma. The interaction energy of two hydrogen atoms is shown on the graph above.
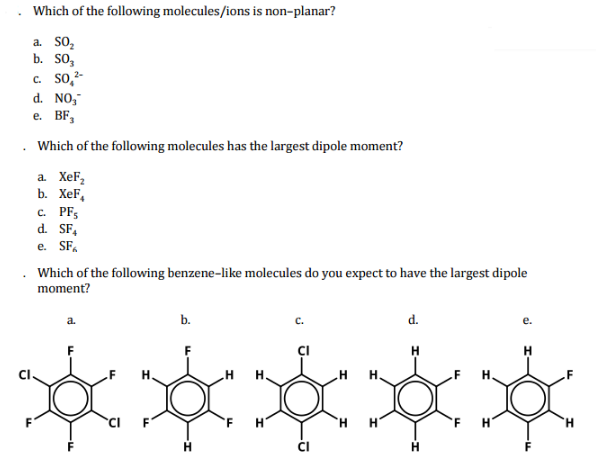 Source: chegg.com
Source: chegg.com
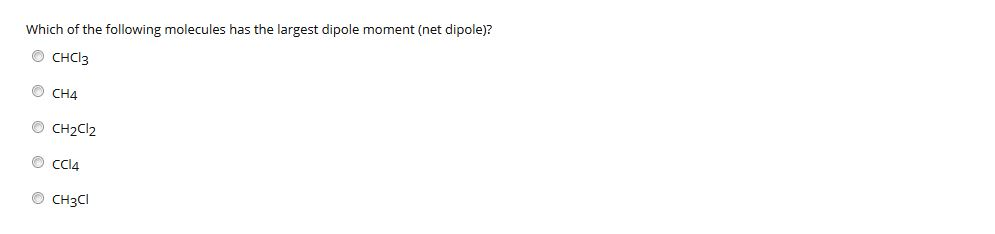
Of the following molecules, which has the largest dipole moment? This is because the individual dipole moments cancel out because of the symmetrical tetrahedral. Of the following molecules, which has the largest dipole moment?
 Source: doubtnut.com
Source: doubtnut.com
Of the following molecules, which has the largest dipole moment? Which bond angle (θ) would result in the maximum dipole moment for the triatomic molecule xy2? (a) chl3 (b) ch4 (c) chcl3 (d) ccl4.
 Source: clutchprep.com
Source: clutchprep.com
A) hf b) hi c) hbr d) hcl e) all of the molecules have the same dipole moment. Of the following molecules the one which has permaanent dipole moment is: The dipole moment in mathematical terms is the product of charge and distance between the atoms.;
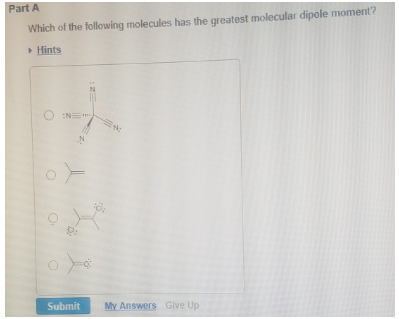 Source: clutchprep.com
Source: clutchprep.com
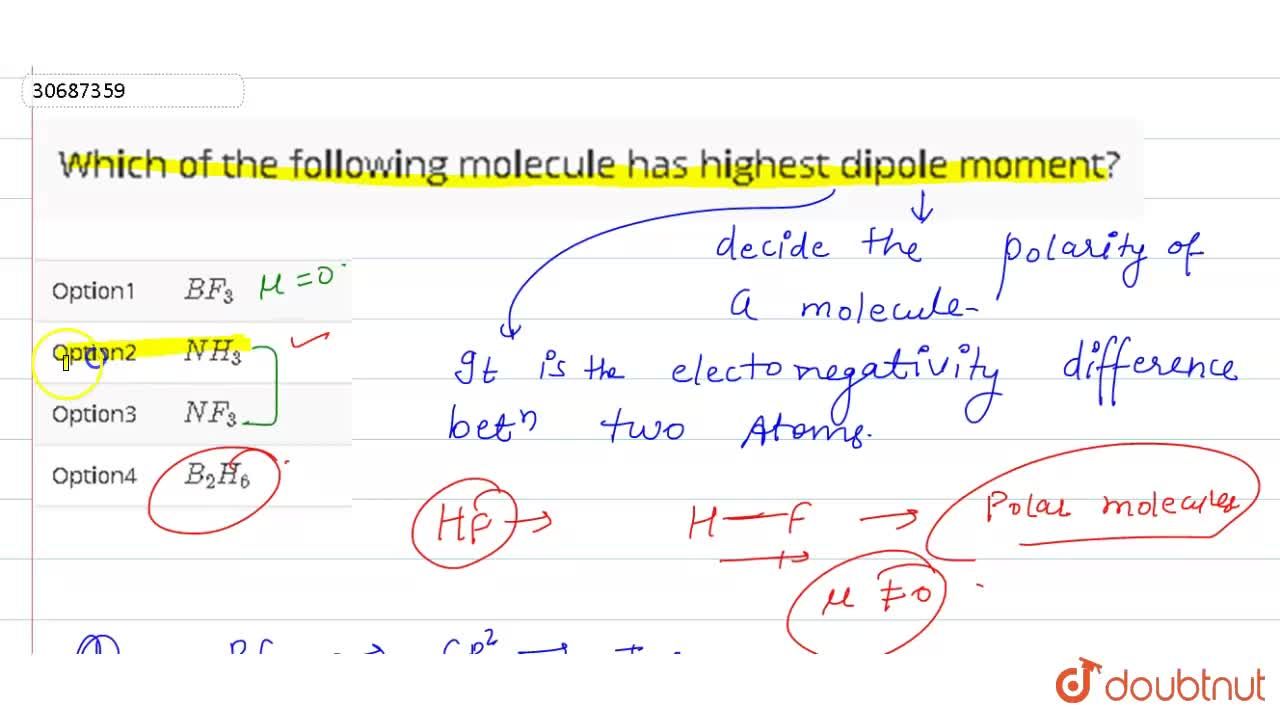
(a) chl3 (b) ch4 (c) chcl3 (d) ccl4. In the given options the dipole moment of pcl_5 is 0.; C ( h) 2 ( c l) 2.
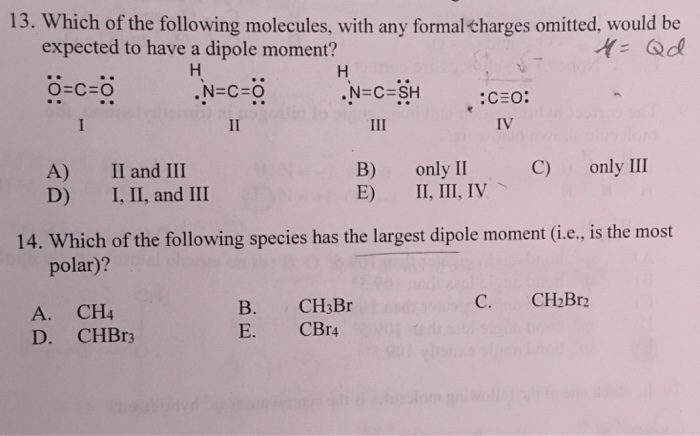
This is because the individual dipole moments cancel out because of the symmetrical tetrahedral. Hf has largest dipole moment because electronegativity difference of both is high so it is highly polar. Of the following molecules, which has the largest dipole moment?
 Source: neetlab.com
Source: neetlab.com
Which bond angle (θ) would result in the maximum dipole moment for the triatomic molecule xy2? C h ( c l) 3. A diatomic molecule has dipole moment of 1.2 d.
 Source: chegg.com
Source: chegg.com
Among the following, the molecule with the highest dipole moment is : In dipole, the shared pair of electron moves towards a more electronegative element.; Should have the highest boiling point?
 Source: slideserve.com
Source: slideserve.com
Whereas in ccl4 a little dipole is reduced due to the subtraction of the individual dipole of a cl present at the top. This is because the individual dipole moments cancel out because of the symmetrical tetrahedral. A stronger intermolecular force result when molecules have permanent dipoles.
 Source: itprospt.com
Source: itprospt.com
If the bond distance is 1.0 a°. C h ( c l) 3. Which of the following covalent bonds has the largest dipole moment?

Asked sep 30, 2016 in chemistry by trollcoma. Click to see full answer. Substance molecular mass (amu) dipole moment (d) propane, ch3ch2ch3 44 0.1 dimethylether, ch3och3 46 1.3 methylchloride, ch3cl 50 1.9 acetaldehyde, ch3cho 44 2.7 acetonitrile, ch3cn 41 3.9 a)ch3cn b)ch3ch2ch3 c)ch3och3 d)ch3cl e)ch3cho 1) 2)of the following substances, only _____ has london dispersion.
 Source: chegg.com
Source: chegg.com
This is because the individual dipole moments cancel out because of the symmetrical tetrahedral. C c l 4 is also called carbon tetrachloride; Among the two and the compound having more dipole moment is nh_3.
 Source: youtube.com
Source: youtube.com
The dipole moment is defined by the charge separated multiplied by the distance (separation) between the charges. C c l 4 is also called carbon tetrachloride; Therefore, c h 2 c l 2 has the highest dipole moment amongst the above three molecules.
 Source: youtube.com
Source: youtube.com
Hydrogen has a lower electronegativity than carbon and carbon is less electronegative than chlorine and so a net dipole moment acts towards the right giving it a high dipole which is 1/2^(1/2) times that of the dipole moment of dichloromethane. The bond dipole moment is a vector quantity since it has both magnitude and direction. The dipole moment is defined by the charge separated multiplied by the distance (separation) between the charges.

The number of pi bonds in the molecule below is. Hf has the largest dipole moment, you can tell which molecule has the largest by looking on the periodic table, they are usually the pair that are furthest from each other and it is also due to them having the biggest difference in electronegativity, usually the closer two elements are, the weaker the dipole moment. C ( h) 2 ( c l) 2.
 Source: doubtnut.com
Source: doubtnut.com
The bond dipole moment is a vector quantity since it has both magnitude and direction. In the given options the dipole moment of pcl_5 is 0.; The number of pi bonds in the molecule below is.
 Source: toppr.com
Source: toppr.com
A) hf b) hi c) hbr d) hcl e) all of the molecules have the same dipole moment. Hf has the largest dipole moment, you can tell which molecule has the largest by looking on the periodic table, they are usually the pair that are furthest from each other and it is also due to them having the biggest difference in electronegativity, usually the closer two elements are, the weaker the dipole moment. (a) chl3 (b) ch4 (c) chcl3 (d) ccl4.
 Source: studylib.net
Source: studylib.net
A) 1 b) 2 c) 3 d) 5 e) 9. A) hf b) hi c) hbr d) hcl e) all of the molecules have the same dipole moment. C ( h) 2 ( c l) 2.
 Source: toppr.com
Source: toppr.com
Which of the following molecules has the largest dipole moment? These are the actual values: Hf has largest dipole moment because electronegativity difference of both is high so it is highly polar.
 Source: meritnation.com
Source: meritnation.com
Among the two and the compound having more dipole moment is nh_3. Hence, only the cl group contributes to the dipole moment of the molecule. Of the following molecules, which has the largest dipole moment?
Also Read :





