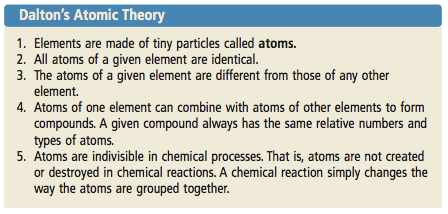
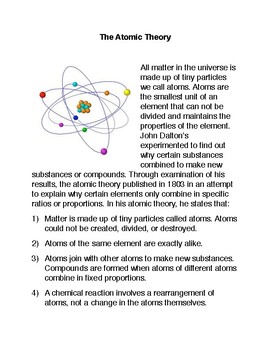
It stated that all matter was made up of small, indivisible particles known as ‘atoms’. All atoms of all elements are the same size.
Daltons Atomic Theory Included Which Idea. 1) all matter is made of atoms. Dalton based his theory on the law of conservation of mass and the law of constant composition. He proposed that the elements are comprised of indestructible atoms, each chemical element possesses a particular kind of atom and all the atoms of a particular element being identical. 1) elements are composed of atoms.
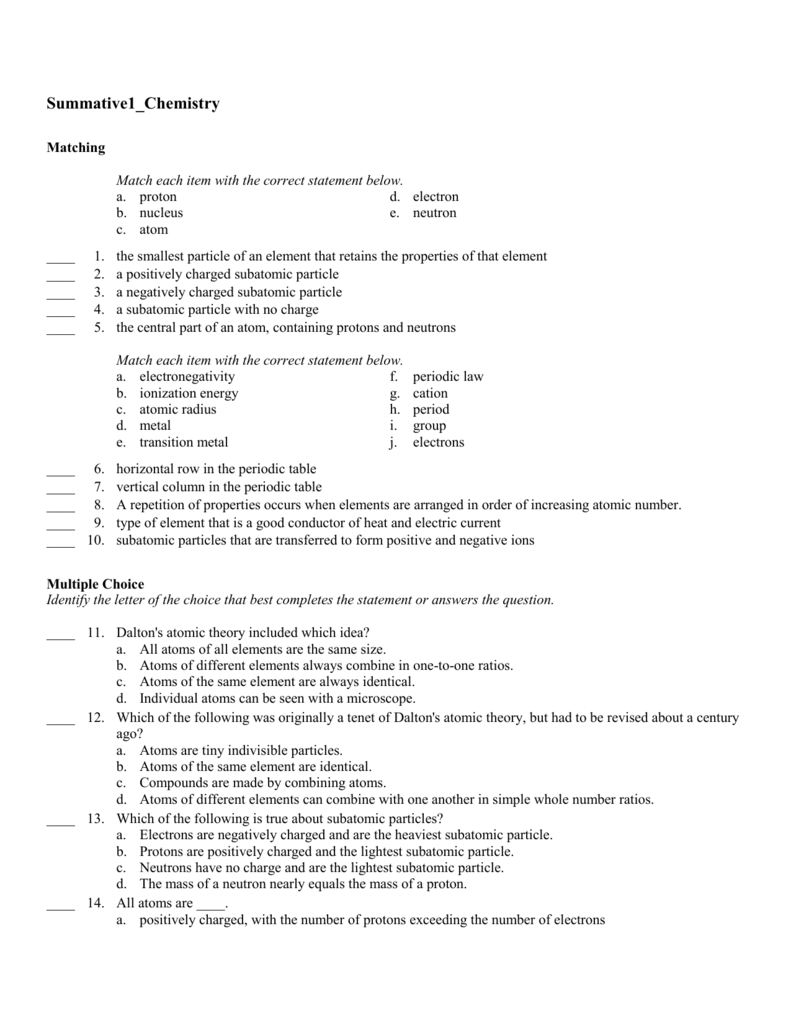 Summative1_Chemistry From studylib.net
Summative1_Chemistry From studylib.net
Related Post Summative1_Chemistry :
In the 16th century, experimentation became popular. In 1803 john dalton published the first comprehensive atomic theory in a new system of chemical philosophy. This is the basic concept which gives the better understanding about many branches of chemistry, both in branches of organic chemistry and branches of inorganic chemistry. John dalton came up with a new atomic theory, which included the following ideas:
Individual atoms can be seen with a microscope.
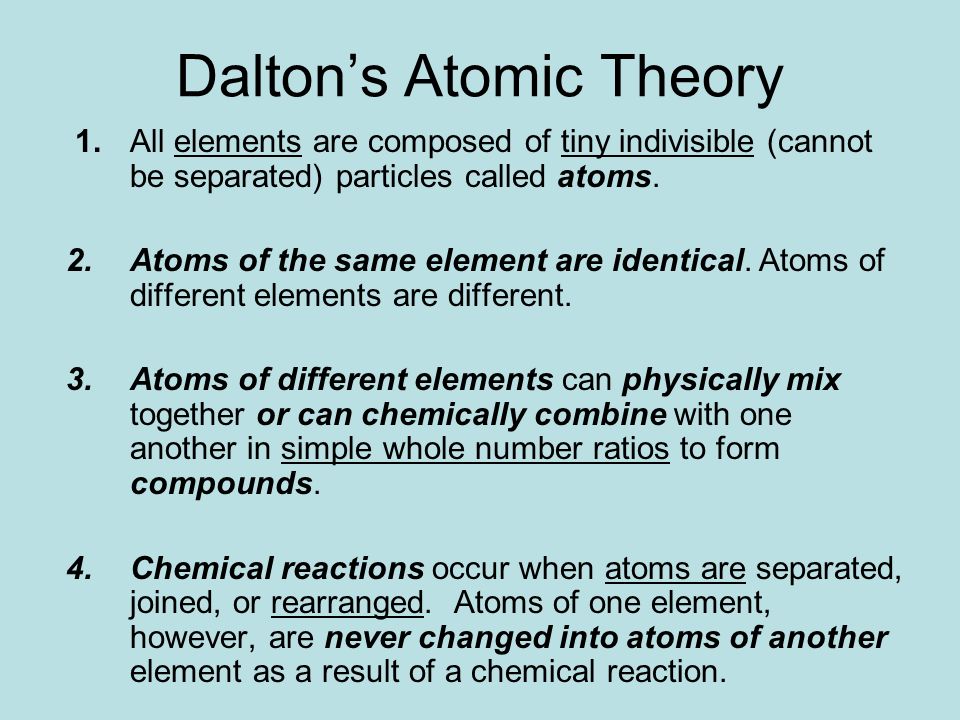
- elements are composed of atoms. (a) matter consists of definite particles, called atoms. One of the popular name in atomic studies is john dalton.he released the postulates of john dalton to describe the atom. His atomic theory helped clarify unexplainable chemical phenomena and inspired scientists that came after him. 1) elements are composed of atoms. 8 how are isobars included in dalton’s atomic theory?
 Source: slideserve.com
Source: slideserve.com
All atoms of all elements are the same size. Dalton�s atomic theory included which idea? His atomic theory helped clarify unexplainable chemical phenomena and inspired scientists that came after him.
 Source: chegg.com
Source: chegg.com
- all matter is made of atoms. He is best known for his work in color blindness, and of course, his atomic theory. Dalton based his theory on the law of conservation of mass and the law of constant composition.
 Source: pinterest.com
Source: pinterest.com
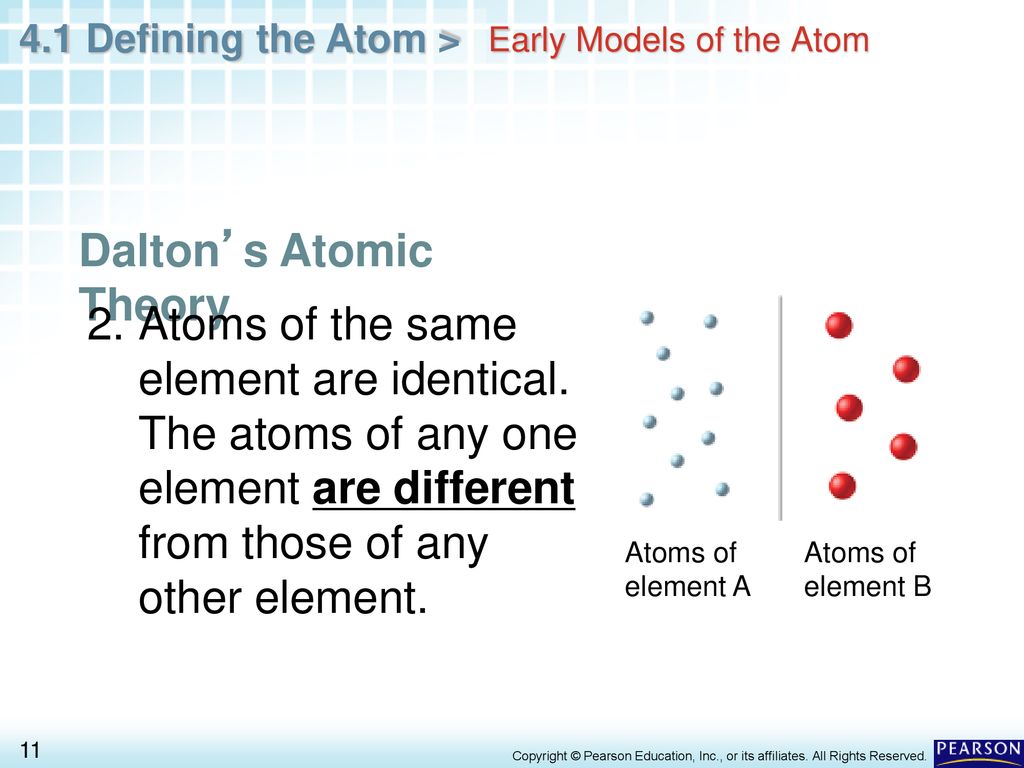
Atoms of the same element are always identical the smallest particle of an element that retains the properties of that element is a(n) ____. The first part of his theory states that all. Atoms of the same element are always identical.
 Source: slidetodoc.com
Source: slidetodoc.com
Individual atoms can be seen with a microscope. Individual atoms can be seen with a microscope. John dalton came up with a new atomic theory, which included the following ideas:
 Source: cpanhd.sitehost.iu.edu
Source: cpanhd.sitehost.iu.edu
Atoms of the same element are always identical. His theory gave us an idea about what the universe is really made up of, and he paved the way for many more important scientific discoveries after he died in 1844. Modern atomic theories evolved from dalton’s basic conclusions.

9 can a dalton atomic theory account for allotropes? 3) elements can mix together 4) atoms only change when mixed with other elements. Atoms of the same element are always identical.
 Source: studylib.net
Source: studylib.net
(a) matter consists of definite particles, called atoms. That all atoms of a certain element were identical, that atoms of one element will have different weights and properties than atoms of another element, that atoms cannot be created or destroyed and that matter is formed by atoms combining in simple whole. He subsequently published a list of known elements and assigned atomic masses.
![Solved] Dalton�s Atomic Theory Includes Which Idea? A. All Atoms Of All Elements Are The Same Size. - Brainly.com](https://us-static.z-dn.net/files/d0a/7ed0a2f120d72aa3b0a57b8b2e177e47.jpg “Solved] Dalton�s Atomic Theory Includes Which Idea? A. All Atoms Of All Elements Are The Same Size. - Brainly.com”) Source: brainly.com
Individual atoms can be seen with a microscope. Individual atoms can be seen with a microscope. That all atoms of a certain element were identical, that atoms of one element will have different weights and properties than atoms of another element, that atoms cannot be created or destroyed and that matter is formed by atoms combining in simple whole.
 Source: chemistryreaders.blogspot.com
Source: chemistryreaders.blogspot.com
Dalton�s atomic theory included which idea? He is best known for his work in color blindness, and of course, his atomic theory. By using experimental methods, dalton transformed democritus�s ideas on atoms into a scientific theory.
 Source: lithub.com
Source: lithub.com
Dalton�s atomic theory included which idea? 9 can a dalton atomic theory account for allotropes? Atoms of the same element are always identical the smallest particle of an element that retains the properties of that element is a(n) ____.
 Source: slideplayer.com
Source: slideplayer.com
Atoms of the same element are always identical. His theory was based on two verified scientific laws: He proposed that the elements are comprised of indestructible atoms, each chemical element possesses a particular kind of atom and all the atoms of a particular element being identical.
 Source: chemistrysaanguyen.weebly.com
Source: chemistrysaanguyen.weebly.com
Atoms of the same element are always identical. Atomic theory is one of the great discovery in the chemistry study. Dalton�s atomic theory was the first complete attempt to describe all matter in terms of atoms and their properties.
 Source: slideplayer.com
Source: slideplayer.com
- atoms of same element are identical, but differ from other elements. All atoms of all elements are the same size. He proposed that the elements are comprised of indestructible atoms, each chemical element possesses a particular kind of atom and all the atoms of a particular element being identical.
 Source: slideplayer.com
Source: slideplayer.com
Dalton�s atomic theory was the first complete attempt to describe all matter in terms of atoms and their properties. Although a schoolteacher, a meteorologist, and an expert on color blindness, john dalton is best known for his pioneering theory of atomism. Atoms of the same element are always identical the smallest particle of an element that retains the properties of that element is a(n) ____.
 Source: slideplayer.com
Source: slideplayer.com
He proposed that the elements are comprised of indestructible atoms, each chemical element possesses a particular kind of atom and all the atoms of a particular element being identical. (a) matter consists of definite particles, called atoms. Dalton published his theory in the early 1800s.
 Source: sites.google.com
Source: sites.google.com
All atoms of all elements are the same size. The first part of his theory states that all. 3) elements can mix together 4) atoms only change when mixed with other elements.
 Source: teacherspayteachers.com
Source: teacherspayteachers.com
By using experimental methods, dalton transformed democritus�s ideas on atoms into a scientific theory. All atoms of all elements are the same size. Dalton�s atomic theory included the idea that:
 Source: en.wikipedia.org
Source: en.wikipedia.org
In 1803 john dalton published the first comprehensive atomic theory in a new system of chemical philosophy. Dalton�s atomic theory included which idea? John dalton’s atomic theory experiment was the first attempt to describe all matter by way of atoms and their properties in a way that was complete.
 Source: studylib.net
Source: studylib.net
His theory was based on two verified scientific laws: Individual atoms can be seen with a microscope. Dalton was born in 1766.
 Source: researchgate.net
Source: researchgate.net
Dalton hypothesized that atoms are indivisible and that all atoms of an element are identical it is now known that atoms are divisible why did j.j. Atoms of the same element are always identical the smallest particle of an element that retains the properties of that element is a(n) ____. One of those parts is a negative tiny particle, which thomson called a.
Also Read :