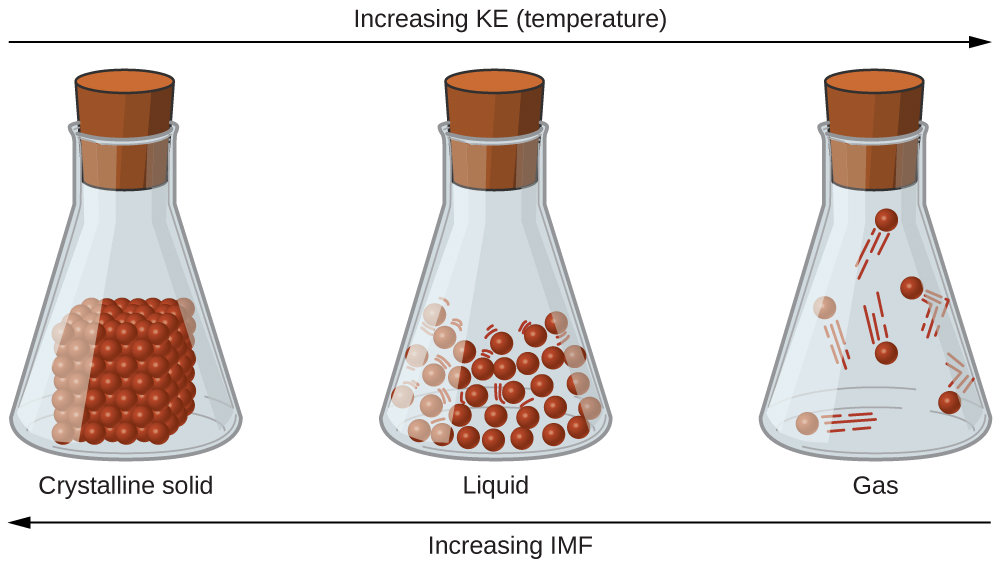
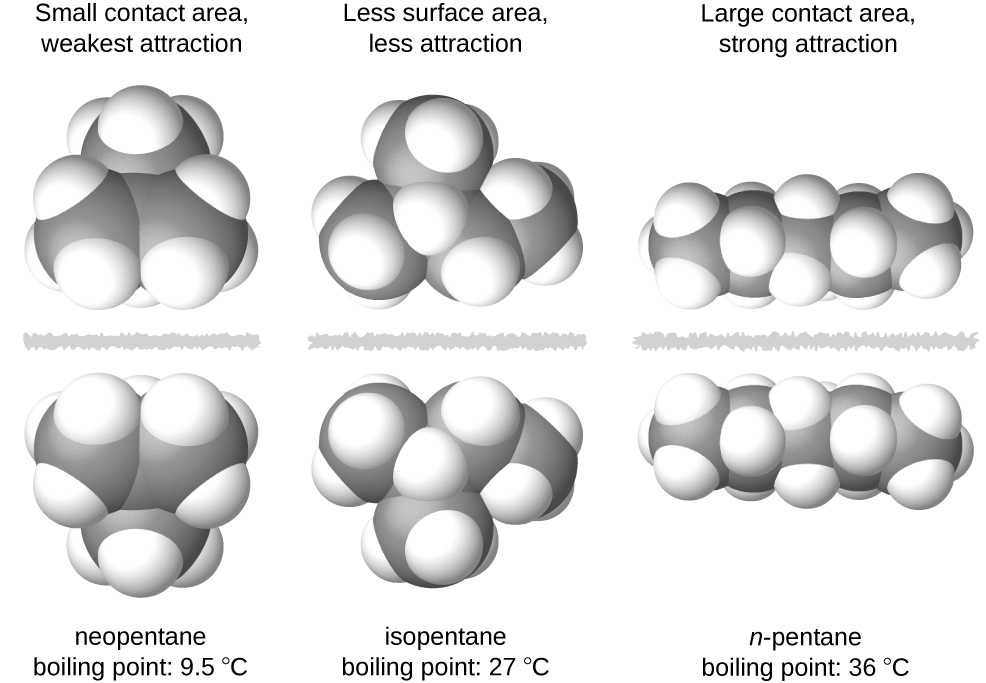
There are three different types of intermolecular forces in terms of strength. The more compact shape of isopentane offers a smaller surface area available for intermolecular contact and, therefore, weaker dispersion forces.
Among The Intermolecular Forces Which Forces Are Typically The Weakest. There are three different types of intermolecular forces in terms of strength. Such intermolecular forces are called van der waals forces and they have nothing to do with the valence electrons. Next the polar covalent bond and the strongest the non polar covalent bond. London dispersion forces in the molecule on the left, areas that have a partial negative charge are pink and areas that have a partial positive charge are blue.
 11.2: Intermolecular Forces - Chemistry Libretexts From chem.libretexts.org
11.2: Intermolecular Forces - Chemistry Libretexts From chem.libretexts.org
Related Post 11.2: Intermolecular Forces - Chemistry Libretexts :
The london dispersion force is a temporary attractive force that results when the electrons in two adjacent atoms occupy positions that make the atoms form temporary dipoles. Among the intermolecular forces, which forces are typically the weakest? And hydrogen has only one electron, therefore is less negative (almost positive in a sense). The bond strengths described range from strongest to weakest (the latter 3 are examples of van der waals forces).
Added 8/26/2018 1:27:49 pm this answer has been confirmed as correct and helpful.
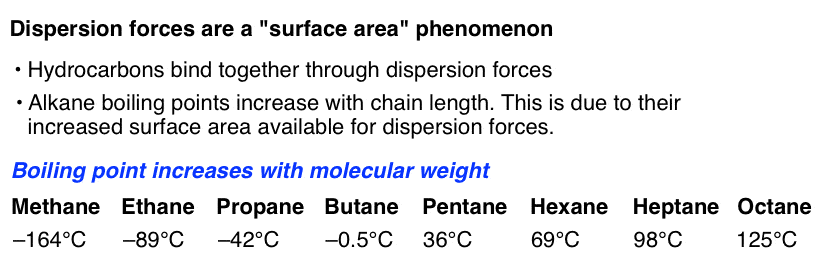
Ionic bondthe weakest of the intramolecular bonds or chemical bonds is the ionic bond. Among the intermolecular forces, london dispersion forces are typically the weakest. There are three different types of intermolecular forces in terms of strength. London dispersion forces, dipole interaction forces, hydrogen bonding is the list among the following choices given in the question that correctly orders intermolecular forces from weakest to strongest. Because compound iii has more branching, these london dispersion forces would be weaker, resulting in a lower boiling point than compound ii. Experts are tested by chegg as specialists in their subject area.
 Source: opentextbc.ca
Source: opentextbc.ca
The bond strengths described range from strongest to weakest (the latter 3 are examples of van der waals forces). Ionic bondthe weakest of the intramolecular bonds or chemical bonds is the ionic bond. London dispersion forces tend to be the weakest intermolecular forces.
 Source: chegg.com
Source: chegg.com
Because compound iii has more branching, these london dispersion forces would be weaker, resulting in a lower boiling point than compound ii. Among the intermolecular forces, which forces are typically the weakest? Among the intermolecular forces, which forces are typically the weakest?
![Solved] 1. Which Among The Intermolecular Forces Is The Weakest? A. Hydrogen Bond B. Ion-Dipole Forces C. Dispersion Forces D. Dipole-Dipole Forces… | Course Hero](https://www.coursehero.com/qa/attachment/18176816/ “Solved] 1. Which Among The Intermolecular Forces Is The Weakest? A. Hydrogen Bond B. Ion-Dipole Forces C. Dispersion Forces D. Dipole-Dipole Forces… | Course Hero”) Source: coursehero.com
The bond strengths described range from strongest to weakest (the latter 3 are examples of van der waals forces). The more compact shape of isopentane offers a smaller surface area available for intermolecular contact and, therefore, weaker dispersion forces. Among the intermolecular forces, which forces are typically the weakest?
 Source: chem.libretexts.org
Source: chem.libretexts.org
London dispersion forces in the molecule on the left, areas that have a partial negative charge are pink and areas that have a partial positive charge are blue. The more compact shape of isopentane offers a smaller surface area available for intermolecular contact and, therefore, weaker dispersion forces. And hydrogen has only one electron, therefore is less negative (almost positive in a sense).

These compounds typically form medium to strong bonds. There are five kinds of intermolecular forces described below; London dispersion forces in the molecule on the left, areas that have a partial negative charge are pink and areas that have a partial positive charge are blue.
 Source: masterorganicchemistry.com
Source: masterorganicchemistry.com
Among the intermolecular forces, london dispersion forces are typically the weakest. These intermolecular forces bind molecules to molecules. 25) option b is answer the london dispersion force or the vander wall forces are the weakest intermolecular force because these are the temporary attractive forces that results when the electrons in two ad.
 Source: brainly.com
Source: brainly.com
What are the strongest and weakest forces of attraction between molecules? Hcl molecules in the nearby attract each other, but the forces of attraction are weak. Among the intermolecular forces, which forces are typically the weakest?

Among the intermolecular forces, which forces are typically the weakest? What are the strongest and weakest forces of attraction between molecules? Among the intermolecular forces, london dispersion forces are typically the weakest.
 Source: doubtnut.com
Source: doubtnut.com
Among the intermolecular forces, which forces are typically the weakest? Neopentane molecules are the most compact of the three, offering the least available surface area for intermolecular contact and, hence, the weakest dispersion forces. And hydrogen has only one electron, therefore is less negative (almost positive in a sense).
 Source: slideplayer.com
Source: slideplayer.com
Compounds ii and iii only exhibit intermolecular london dispersion forces, so they would be the two lowest boiling compounds (weakest intermolecular forces). Y�s molecules experience stronger london dispersion forces than x�s molecules. Among the intermolecular forces, which forces are typically the weakest?
 Source: study.com
Source: study.com
These compounds typically form medium to strong bonds. Y�s molecules experience stronger london dispersion forces than x�s molecules. There are five kinds of intermolecular forces described below;
![Solved] 1. Which Among The Intermolecular Forces Is The Weakest? A. Hydrogen Bond B. Ion-Dipole Forces C. Dispersion Forces D. Dipole-Dipole Forces… | Course Hero](https://www.coursehero.com/qa/attachment/18176805/ “Solved] 1. Which Among The Intermolecular Forces Is The Weakest? A. Hydrogen Bond B. Ion-Dipole Forces C. Dispersion Forces D. Dipole-Dipole Forces… | Course Hero”) Source: coursehero.com
Among the intermolecular forces, which forces are typically the weakest? These are the weakest of the intermolecular forces and exist between all types of molecules, whether ionic or covalent—polar or nonpolar. The london dispersion force is the weakest intermolecular force.
 Source: opentextbc.ca
Source: opentextbc.ca
Among the intermolecular forces, which forces are typically the weakest? These are the weakest of the intermolecular forces and exist between all types of molecules, whether ionic or covalent—polar or nonpolar. What are the strongest and weakest forces of attraction between molecules?
 Source: khanacademy.org
Source: khanacademy.org
London dispersion forces which statement best describes the effect of low lonization energies and low electronegativities on metallic bonding? Hcl molecules in the nearby attract each other, but the forces of attraction are weak. London dispersion forces which statement best describes the effect of low lonization energies and low electronegativities on metallic bonding?

Ionic bondthe weakest of the intramolecular bonds or chemical bonds is the ionic bond. Experts are tested by chegg as specialists in their subject area. Because compound iii has more branching, these london dispersion forces would be weaker, resulting in a lower boiling point than compound ii.
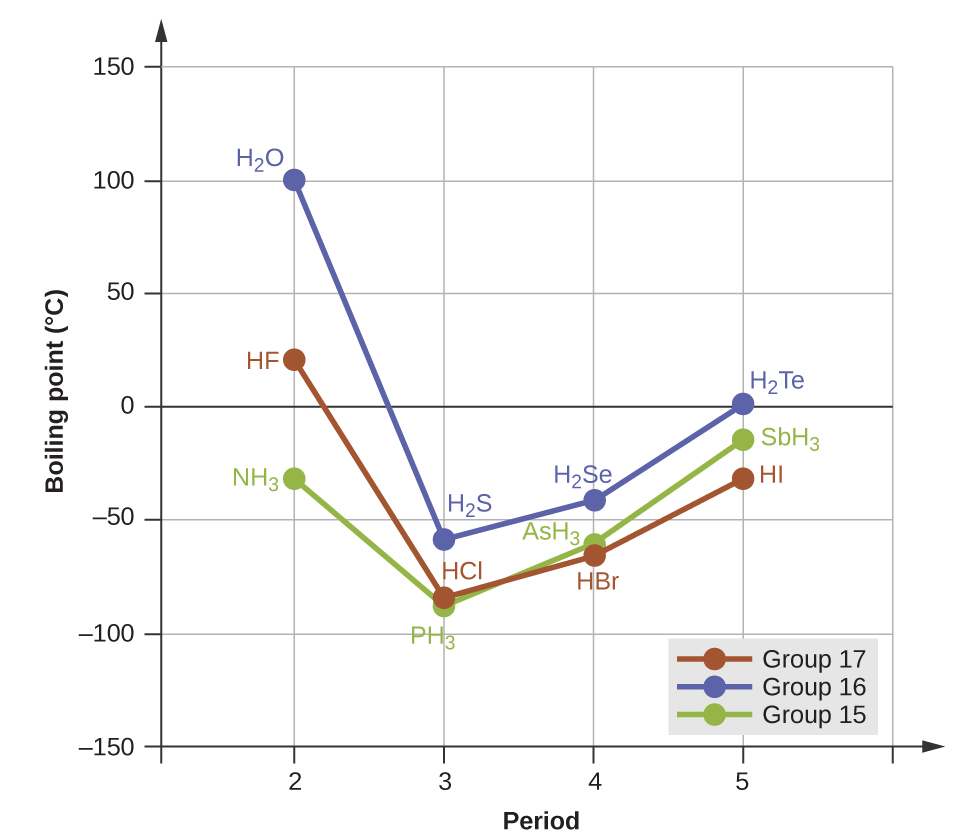 Source: opentextbc.ca
Source: opentextbc.ca
The london dispersion force is a temporary attractive force that results when the electrons in two adjacent atoms occupy positions that make the atoms form temporary dipoles. Neopentane molecules are the most compact of the three, offering the least available surface area for intermolecular contact and, hence, the weakest dispersion forces. London dispersion forces in the molecule on the left, areas that have a partial negative charge are pink and areas that have a partial positive charge are blue.
 Source: scientifictutor.org
Source: scientifictutor.org
Added 8/26/2018 1:27:49 pm this answer has been confirmed as correct and helpful. We review their content and use your feedback to keep the quality high. These compounds typically form medium to strong bonds.
 Source: khanacademy.org
Source: khanacademy.org
These intermolecular forces bind molecules to molecules. These compounds typically form medium to strong bonds. Y�s molecules experience stronger london dispersion forces than x�s molecules.
 Source: slideplayer.com
Source: slideplayer.com
London dispersion forces, dipole interaction forces, hydrogen bonding is the list among the following choices given in the question that correctly orders intermolecular forces from weakest to strongest. There are three different types of intermolecular forces in terms of strength. Because compound iii has more branching, these london dispersion forces would be weaker, resulting in a lower boiling point than compound ii.
 Source: chem.libretexts.org
Source: chem.libretexts.org
Among the intermolecular forces, which forces are typically the weakest? Among the intermolecular forces, which forces are typically the weakest? Among the intermolecular forces, which forces are typically the weakest?
Also Read :