Mesomers are a type of compounds in which net rotation of plane polarised light is zero. There are several types of achiral stationary phases currently available for the separation of achiral compounds.
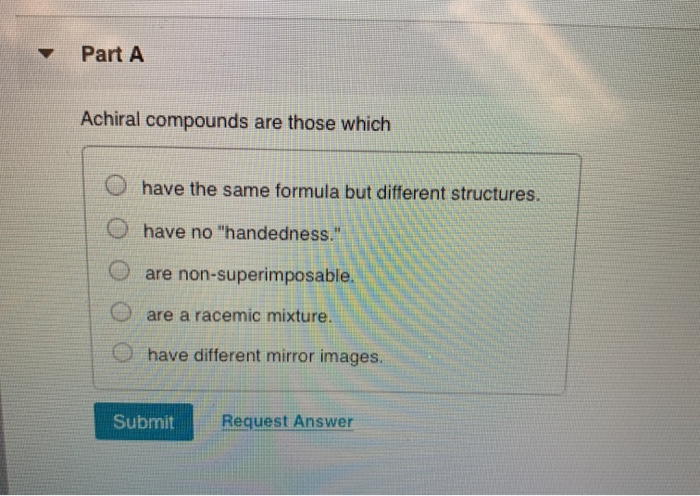
Achiral Compounds Are Those Which. All pure achiral compounds are optically inactive. Structurally similar (either with racemic compound or resolving agent) achiral compound.14 it is possible that the recrystallization from an adequate solvent does not give pure enantiomers, but a good enrichment can be of ten achieved by fractionated precipitation. This geometric property is called chirality. Investigations of isomeric tartaric acid salts, carried out by louis pasteur in the mid 19th century, were instrumental in elucidating some of the subtleties of stereochemistry.
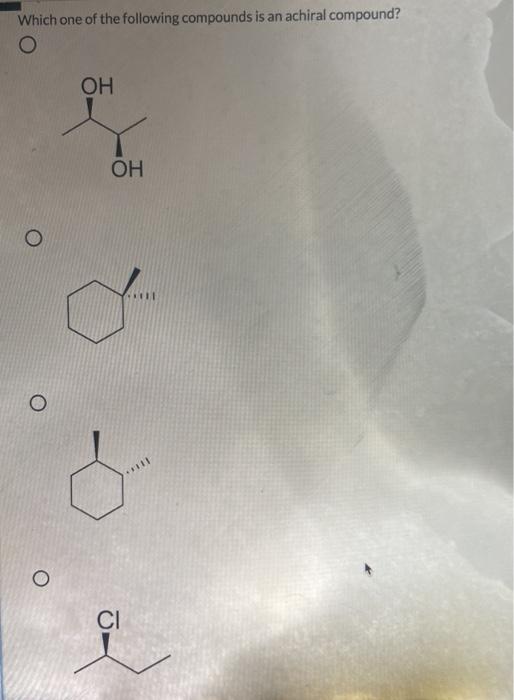
Related Post Solved Which One Of The Following Compounds Is An Achiral | Chegg.com :
Since our compounds are primarily basic, we chose to prescreen using the following stationary phases: All pure achiral compounds are optically inactive. I.e to be simple , mesomers are type of organic compounds where two chiral carbons are present and those two are similar , so net rotation is zero. And has this lone parents in this, like, weird state of it really can�t be a cairo center.
Meso compound an achiral compound possessing two or more chiral centers that also has chiral isomers
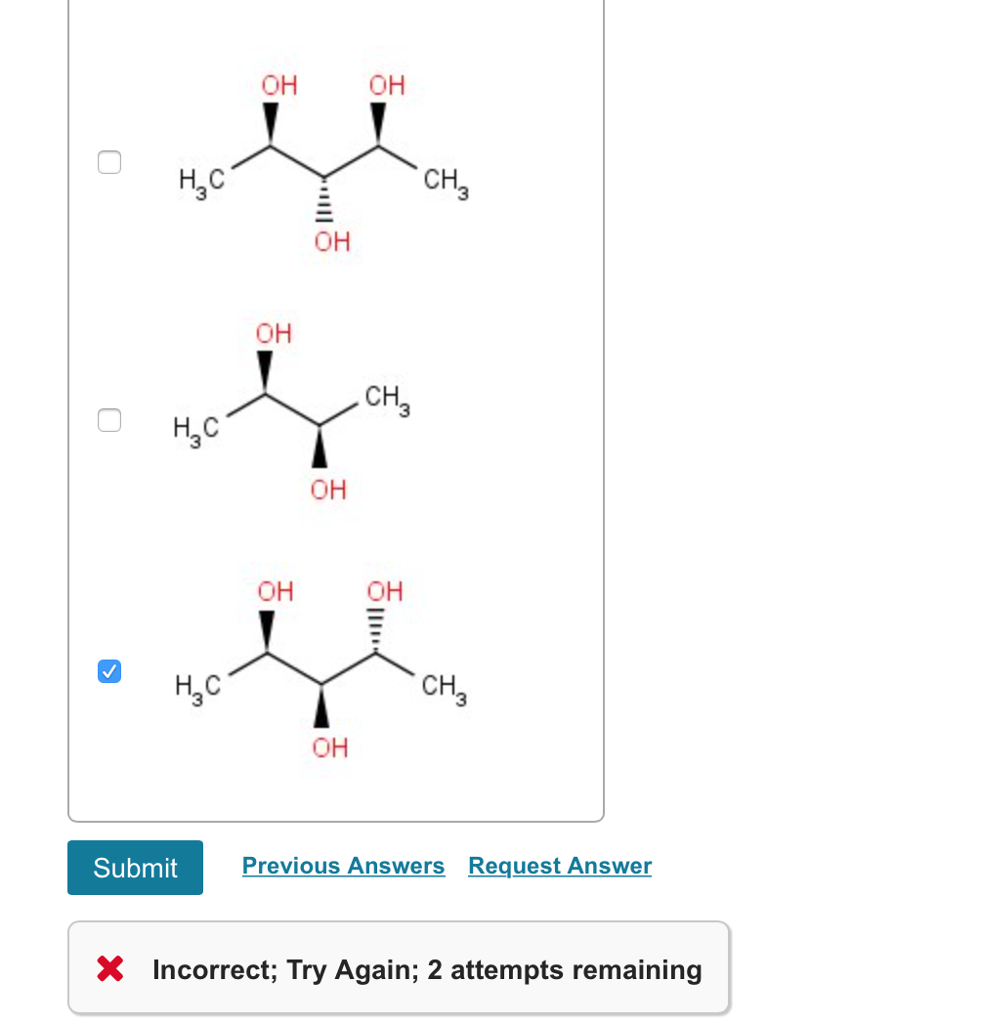
I.e to be simple , mesomers are type of organic compounds where two chiral carbons are present and those two are similar , so net rotation is zero. In chemistry, a molecule or ion is called chiral (/ k aɪ ˈ r æ l /) if it cannot be superposed on its mirror image by any combination of rotations, translations, and some conformational changes. Which of the following compounds are chiral? A meso compound is an achiral compound that has chiral centers. Achiral compounds and meso compounds are related to each other in some properties but have some different properties as well. Part a achiral compounds are those which have the same formula but different structures.
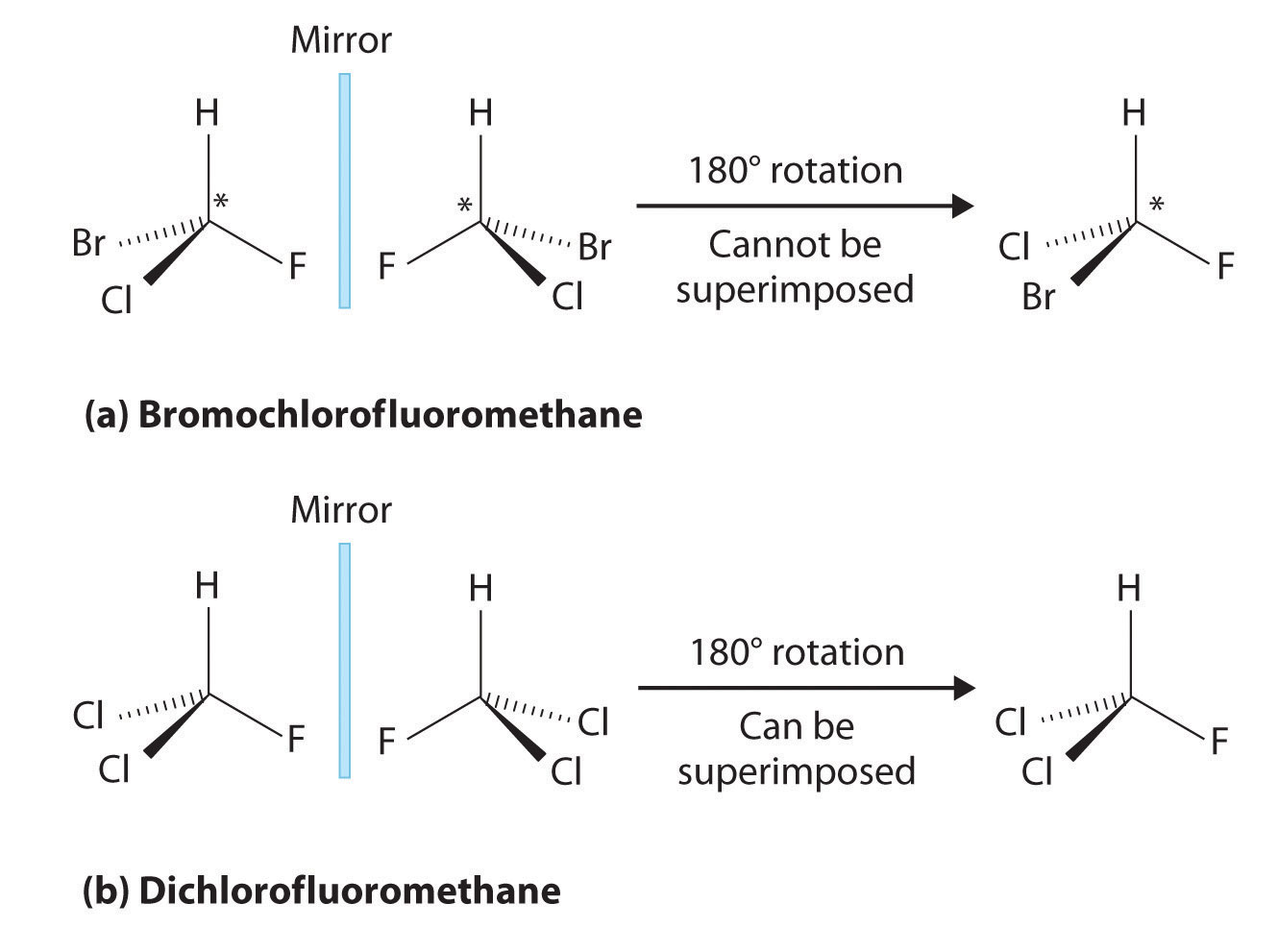 Source: chem.libretexts.org
Source: chem.libretexts.org
In summary, an achiral compound is the opposite of a chiral compound. Achiral compounds and meso compounds are related to each other in some properties but have some different properties as well. So we can�t really say those air cairo centers the nitrogen, since it only has three.
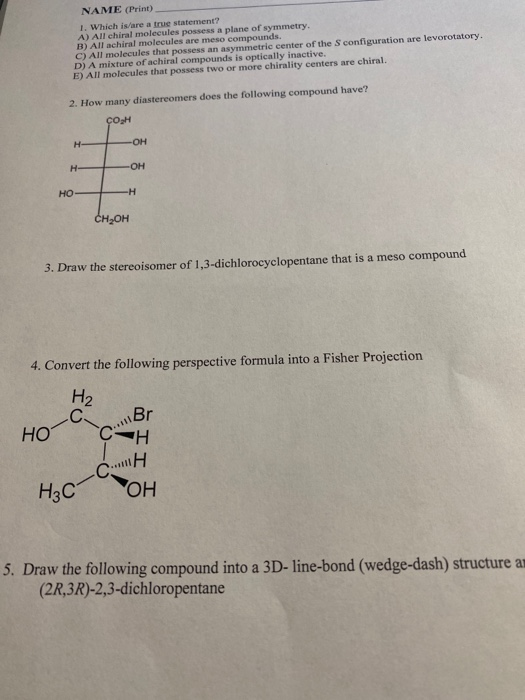 Source: chegg.com
Source: chegg.com
Pyridine, deap, amino, nitro and cyano. However, as you suggest, chair flip of b gives the mirror image of the original drawing, which means the original and mirror image are equivalent, assuming. Chiral compound do not superimpose.
 Source: chegg.com
Source: chegg.com
Thus, 1 is optically inactive. I.e to be simple , mesomers are type of organic compounds where two chiral carbons are present and those two are similar , so net rotation is zero. This problem has been solved!
 Source: numerade.com
Source: numerade.com
The key difference between the terms achiral and meso is that achiral compounds have no chiral centers whereas meso compounds have multiple chiral centers. A meso compound is an achiral compound that has chiral centers. This problem has been solved!
 Source: chegg.com
Source: chegg.com
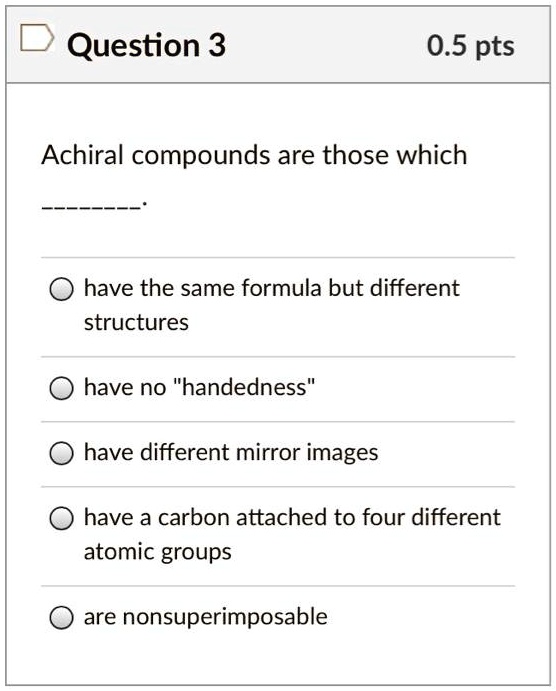
- achiral compounds are those which _____. And then all the carbons out here. Meso compounds are achiral (optically inactive) diastereomers of chiral stereoisomers.
 Source: study.com
Source: study.com
Two naturally occurring forms of tartaric acid are optically inactive. Meso compound an achiral compound possessing two or more chiral centers that also has chiral isomers Mesomers are a type of compounds in which net rotation of plane polarised light is zero.
 Source: chegg.com
Source: chegg.com
Some molecules are achiral even though they contain chirality centers. Pyridine, deap, amino, nitro and cyano. Mesomers are a type of compounds in which net rotation of plane polarised light is zero.
 Source: socratic.org
Source: socratic.org
All pure achiral compounds are optically inactive. 11) achiral compounds are those which _____. Mesomers are a type of compounds in which net rotation of plane polarised light is zero.
 Source: pediaa.com
Source: pediaa.com
D) have the same formula but different structures. This problem has been solved! And has this lone parents in this, like, weird state of it really can�t be a cairo center.
 Source: sciencedirect.com
Source: sciencedirect.com
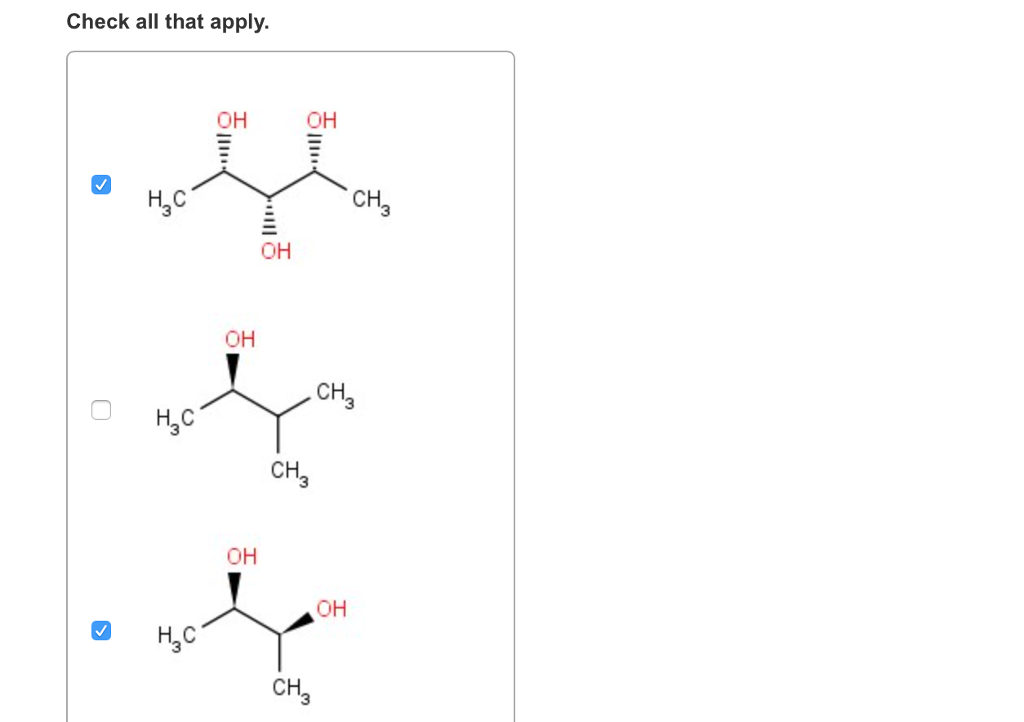
E) have a carbon attached to four different atoms. Choose the achiral structures choose all the achiral compounds among the ones shown. I.e to be simple , mesomers are type of organic compounds where two chiral carbons are present and those two are similar , so net rotation is zero.
 Source: chegg.com
Source: chegg.com
A meso compound is an achiral compound that has chiral centers. There are several types of achiral stationary phases currently available for the separation of achiral compounds. And then all the carbons out here.
 Source: chegg.com
Source: chegg.com
A meso compound is an achiral compound that has chiral centers. Chiral surfaces are critical components of enantioselective heterogeneous processes such as those used to prepare enantiomerically pure pharmaceuticals. Compound c has a plane of symmetry, so it is clearly achiral.
 Source: chegg.com
Source: chegg.com
Meso compounds are achiral (optically inactive) diastereomers of chiral stereoisomers. Hydrogen is attached to them. In summary, an achiral compound is the opposite of a chiral compound.
 Source: socratic.org
Source: socratic.org
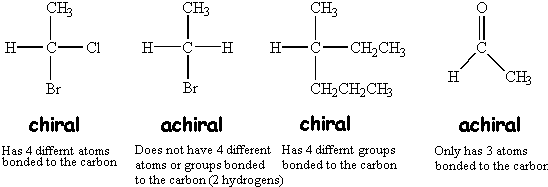
Pyridine, deap, amino, nitro and cyano. A chiral carbon or asymmetric carbon atom is a carbon atom that is attached to four different types of atoms or groups of atoms. Compound c has a plane of symmetry, so it is clearly achiral.
 Source: chegg.com
Source: chegg.com
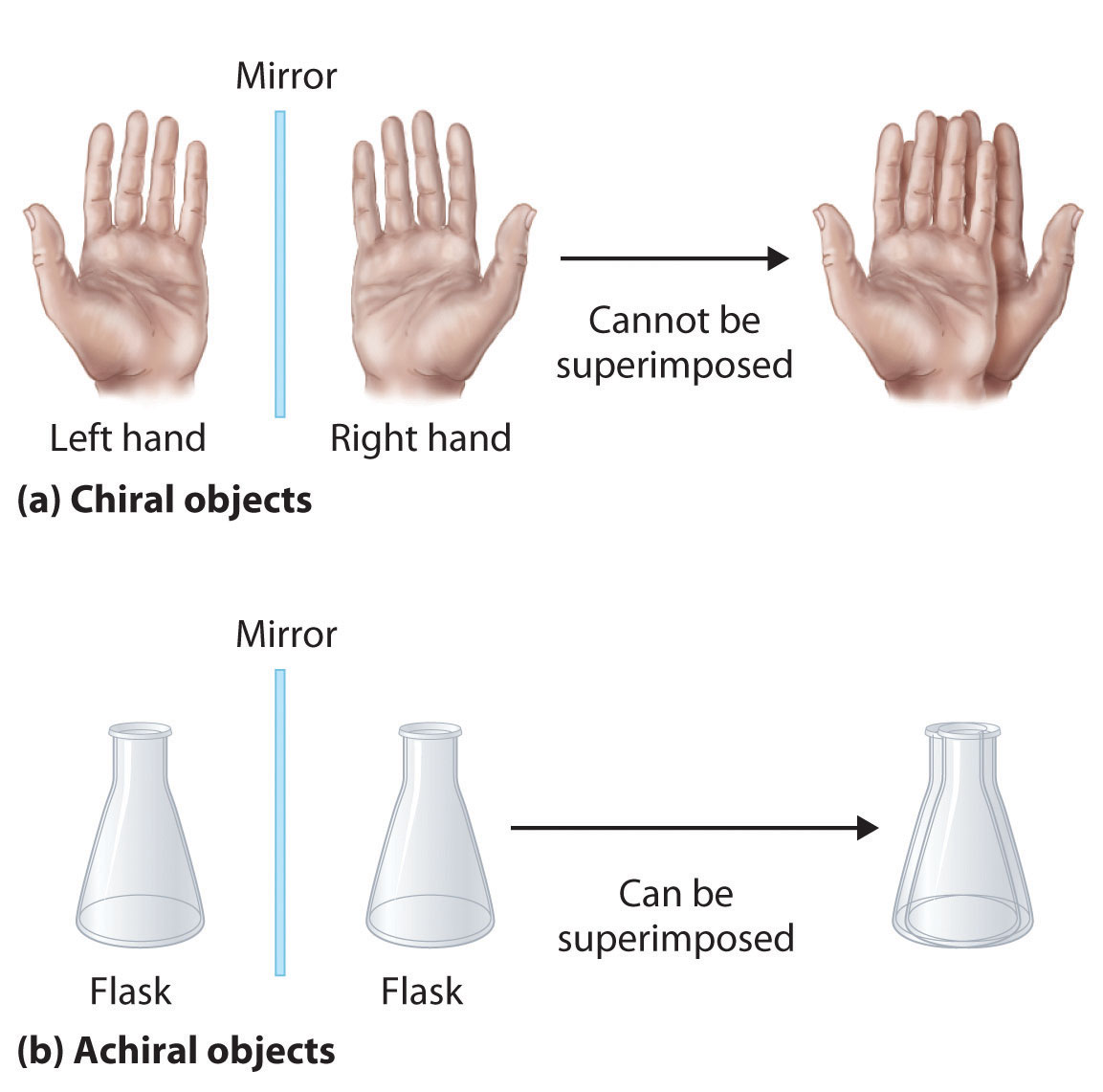
A meso compound is an achiral compound that has chiral centers. And then all the carbons out here. Achiral (not chiral) objects are those objects that are identical to their mirror image.
 Source: sciencedirect.com
Source: sciencedirect.com
However, as you suggest, chair flip of b gives the mirror image of the original drawing, which means the original and mirror image are equivalent, assuming. Thus, 1 is optically inactive. Optically active compounds are those which can rotate the plane of plane polarised light, and those which not are optically inactive compounds.
 Source: chegg.com
Source: chegg.com
Structurally similar (either with racemic compound or resolving agent) achiral compound.14 it is possible that the recrystallization from an adequate solvent does not give pure enantiomers, but a good enrichment can be of ten achieved by fractionated precipitation. Compound c has a plane of symmetry, so it is clearly achiral. The key difference between the terms achiral and meso is that achiral compounds have no chiral centers whereas meso compounds have multiple chiral centers.
 Source: youtube.com
Source: youtube.com
- achiral compounds are those which _____. [‚ȯr·ē·ən′tā·shən i‚fekt] (electricity) those bulk properties of a material which result from orientation polarization. I.e to be simple , mesomers are type of organic compounds where two chiral carbons are present and those two are similar , so net rotation is zero.
 Source: study.com
Source: study.com
Which of the following compounds are chiral? All pure achiral compounds are optically inactive. Meso compounds are achiral (optically inactive) diastereomers of chiral stereoisomers.

- stereoisomers that are nonsuperimposable mirror images of each other are known as _____. And has this lone parents in this, like, weird state of it really can�t be a cairo center. See the answer see the answer see the answer done loading.
Also Read :





